Page 39 - Radiochemistry and nuclear chemistry
P. 39
28 Radiochemistry and Nuclear Chemistry
TRANSITION STATE ~#
o=
>.
uJ E;
z
Ill
..!
<_ PRODUCTS AB + C
I- aN= AN t
Z ul
I.-
REACTANTS A +
REACTION COORDINATE
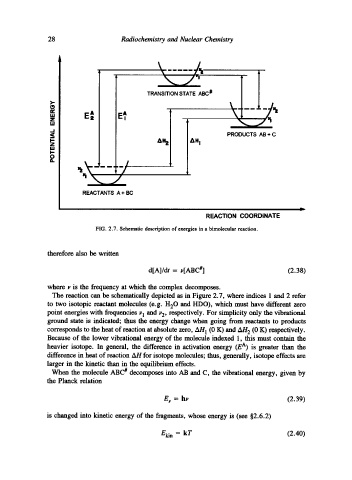
FIG. 2.7. Schematic description of energies in a bimolecular reaction.
therefore also be written
d[A]/dt = p[ABC #] (2.38)
where J, is the frequency at which the complex decomposes.
The reaction can be schematically depicted as in Figure 2.7, where indices 1 and 2 refer
to two isotopic reactant molecules (e.g. H20 and HDO), which must have different zero
point energies with frequencies J'l and ~2, respectively. For simplicity only the vibrational
ground state is indicated; thus the energy change when going from reactants to products
corresponds to the heat of reaction at absolute zero, AH 1 (0 K) and AH 2 (0 K) respectively.
Because of the lower vibrational energy of the molecule indexed 1, this must contain the
heavier isotope. In general, the difference in activation energy (E A) is greater than the
difference in heat of reaction AH for isotope molecules; thus, generally, isotope effects are
larger in the kinetic than in the equilibrium effects.
When the molecule ABC # decomposes into AB and C, the vibrational energy, given by
the Planck relation
Ep = hi, (2.39)
is changed into kinetic energy of the fragments, whose energy is (see w
Eki n = kT (2.40)