Page 37 - Radiochemistry and nuclear chemistry
P. 37
26 Radiochemistry and Nuclear Chemistry
25
o~
j o.O. 9
e # ,=.,, .:\
-
\ .~. 9 -,, :
~ 9 , 9 .
.
i : : : \ ~ :
,"
% ".- : : -- \'- \ -- 9 1 . : :
4-/ 9 9 -- i ; ' 9 ; d. 9
o 20
.~. i '~- 9149 '~ :
I
I.
: : ',! : :
15 I I I I
0 10 20 30 40
Radius (square root of sum of sample weights, g)
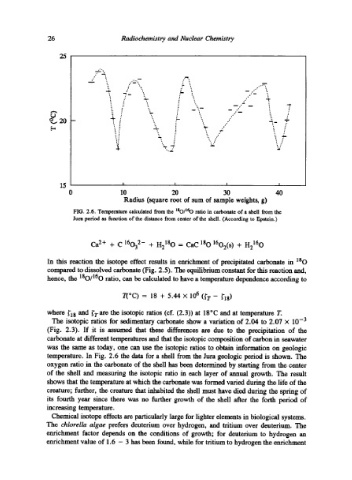
FIG. 2.6. Temperature calculated from the lSO/160 ratio in carbonate of a shell from the
Jura period as function of the distance from center of the shell. (According to Epstein.)
Ca 2+ + C16032- + H218 0 = CaC18 O1602(s ) + H216 0
In this reaction the isotope effect results in enrichment of precipitated carbonate in 180
compared to dissolved carbonate (Fig. 2.5). The equilibrium constant for this reaction and,
hence, the 180/160 ratio, can be calculated to have a temperature dependence according to
T(~ = 18 + 5.44 x 10 6 (~'T- ~'18)
where ~'18 and ~'T are the isotopic ratios (of. (2.3)) at 18~ and at temperature T.
The isotopic ratios for sedimentary carbonate show a variation of 2.04 to 2.07 x 10 -3
(Fig. 2.3). If it is assumed that these differences are due to the precipitation of the
carbonate at different temperatures and that the isotopic composition of carbon in seawater
was the same as today, one can use the isotopic ratios to obtain information on geologic
temperature. In Fig. 2.6 the data for a shell from the Jura geologic period is shown. The
oxygen ratio in the carbonate of the shell has been determined by starting from the center
of the shell and measuring the isotopic ratio in each layer of annual growth. The result
shows that the temperature at which the carbonate was formed varied during the life of the
creature; further, the creature that inhabited the shell must have died during the spring of
its fourth year since there was no further growth of the shell after the forth period of
increasing temperature.
Chemical isotope effects are particularly large for lighter elements in biological systems.
The chlorella algae prefers deuterium over hydrogen, and tritium over deuterium. The
enrichment factor depends on the conditions of growth; for deuterium to hydrogen an
enrichment value of 1.6 - 3 has been found, while for tritium to hydrogen the enrichment