Page 33 - Radiochemistry and nuclear chemistry
P. 33
22 Radiochemistry and Nuclear Chemistry
2.6.2. Kinetic energy and temperature
The kinetic energy of one mole of an ideal gas at temperature T (K) is given by its
translational energy, which according to the kinetic theory of gases is
Etr = 3RT/2 (J mole- 1) (2.20)
Dividing by the Avogadro number N A yields the average kinetic energy per molecule (or
particle)
bTtr = 3kT/2 (J particle-l) (2.21)
where k = R/N A. From mechanics we know that the kinetic energy of a single particle of
mass m and velocity v is
Elfi n = t/2mv 2 (2.22)
Summing over a large number of particles, we can defme an average kinetic energy
m
Elfin = ~AmO 2 (2.23)
where ~ 2 is the mean square velocity. Because_ (2.21) is the average kinetic energy at
temperature T, it must equal (2.23), i.e. Etr = Elfin, thus
3kT/2 = ~,~mp 2 (2.24)
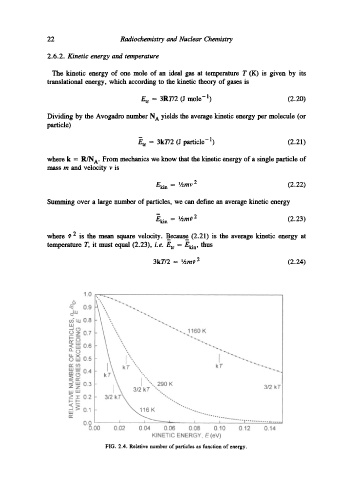
FIG. 2.4. Relative number of particles as function of energy.