Page 30 - Radiochemistry and nuclear chemistry
P. 30
Nuclei, Isotopes and Isotope Separation 19
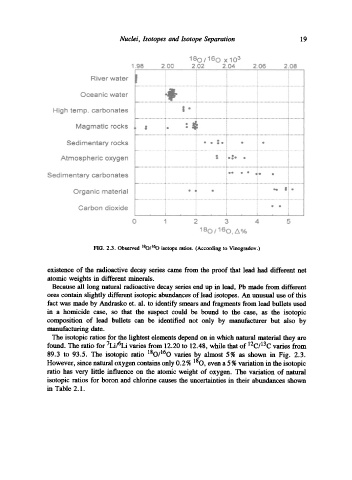
FIG. 2.3. Observed ~SO/l~O isotope ratios. (According to Vinogradov.)
existence of the radioactive decay series came from the proof that lead had different net
atomic weights in different minerals.
Because all long natural radioactive decay series end up in lead, Pb made from different
ores contain slightly different isotopic abundances of lead isotopes. An unusual use of this
fact was made by Andrasko et. al. to identify smears and fragments from lead bullets used
in a homicide case, so that the suspect could be bound to the case, as the isotopic
composition of lead bullets can be identified not only by manufacturer but also by
manufacturing date.
The isotopic ratios for the lightest dements depend on in which natural material they are
found. The ratio for 7Li/6Li varies from 12.20 to 12.48, while that of 12C/13C varies from
89.3 to 93.5. The isotopic ratio 180/160 varies by almost 5% as shown in Fig. 2.3.
However, since natural oxygen contains only 0.2 % 180, even a 5 % variation in the isotopic
ratio has very little influence on the atomic weight of oxygen. The variation of natural
isotopic ratios for boron and chlorine causes the uncertainties in their abundances shown
in Table 2.1.