Page 53 - Radiochemistry and nuclear chemistry
P. 53
42 Radiochemistry and Nuclear Chemistry
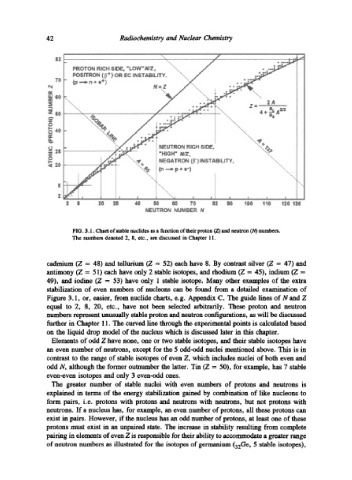
FIG. 3.1. Chart of stable nuclides as a function of their proton (Z) and neutron (N) numbers.
The numbers denoted 2, 8, etc., are discussed in Chapter 11.
cadmium (Z = 48) and tellurium (Z = 52) each have 8. By contrast silver (Z = 47) and
antimony (Z = 51) each have only 2 stable isotopes, and rhodium (Z = 45), indium (Z =
49), and iodine (Z = 53) have only 1 stable isotope. Many other examples of the extra
stabilization of even numbers of nucleons can be found from a detailed examination of
Figure 3.1, or, easier, from nuclide charts, e.g. Appendix C. The guide lines of N and Z
equal to 2, 8, 20, etc., have not been selected arbitrarily. These proton and neutron
numbers represent unusually stable proton and neutron configurations, as will be discusseA
further in Chapter 11. The curved line through the experimental points is calculated based
on the liquid drop model of the nucleus which is discussed later in this chapter.
Elements of odd Z have none, one or two stable isotopes, and their stable isotopes have
an even number of neutrons, except for the 5 odd-odd nuclei mentioned above. This is in
contrast to the range of stable isotopes of even Z, which includes nuclei of both even and
odd N, although the former outnumber the latter. Tin (Z = 50), for example, has 7 stable
even-even isotopes and only 3 even-odd ones.
The greater number of stable nuclei with even numbers of protons and neutrons is
explained in terms of the energy stabilization gained by combination of like nucleons to
form pairs, i.e. protons with protons and neutrons with neutrons, but not protons with
neutrons. If a nucleus has, for example, an even number of protons, all these protons can
exist in pairs. However, if the nucleus has an odd number of protons, at least one of these
protons must exist in an unpaired state. The increase in stability resulting from complete
pairing in elements of even Z is responsible for their ability to accommodate a greater range
of neutron numbers as illustrated for the isotopes of germanium (32Ge, 5 stable isotopes),