Page 57 - Radiochemistry and nuclear chemistry
P. 57
46 Radiochemistry and Nuclear Chemistry
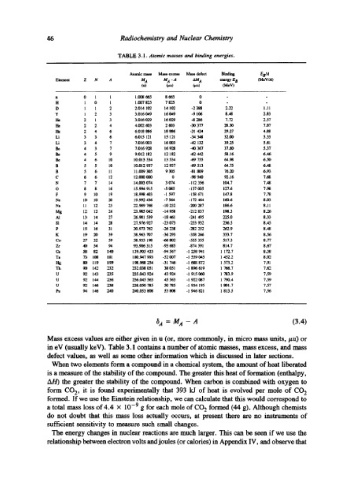
TABLE 3.1. Atomic masses and binding energies.
Atomic mass Mass exor, aa Mass defect Binding EB/A
~-~n=m z N .4 u z M z -,4 aM z ~,,~,~ tr B (M~v/,1)
(u) (~u) (~u) (M 9
n 0 1 1 1.008 665 8 665 0 - -
H 1 0 1 1.007 825 7 825 0 - -
D 1 1 2 2.014 102 14 102 -2 388 2.22 1.11
T 1 2 3 3.016 049 16 049 -9 106 8.48 2.83
He 2 1 3 3.016 029 16 029 -8 286 7.72 2.57
He 2 2 4 4.1302 603 2 603 -30 377 28.30 7.07
He 2 4 6 6.018 886 18 886 -31 424 29.27 4.88
Li 3 3 6 6.015 121 15 121 -34 348 32.00 5.33
Li 3 4 7 7.016 003 16 003 -42 132 39.25 5.61
Be 4 3 7 7.016 928 16 928 -40 367 37.60 5.37
Be 4 5 9 9.012 182 12 182 -62 442 58.16 6.46
Be 4 6 10 10.013 534 13 534 -69 755 64.98 6.50
B 5 5 10 10.012 937 12 937 -69 513 64.75 6.48
B 5 6 11 11.009 305 9 305 -81 809 76.20 6.93
C 6 6 12 12.000 000 0 -98 940 92.16 7.68
N 7 7 14 14.003 074 3 074 -112 356 104.7 7.48
O 8 8 16 15.994 915 -5 085 -137 005 127.6 7.98
F 9 10 19 18.998 403 -1 597 -158 671 147.8 7.78
Ne 10 10 20 19.992 436 -7 564 -172 464 160.6 8.03
Na 11 12 23 22.989 768 -10 232 -200 287 186.6 8.11
Mg 12 12 24 23.985 042 -14 958 -212 837 198.3 8.26
AI 13 14 27 26.981 539 -18 461 -241 495 225.0 8.33
Si 14 14 28 27.976 927 -23 073 -253 932 236.5 8.45
P 15 16 31 30.973 762 -26 238 -282 252 262.9 8.48
K 19 20 39 38.963 707 -36 293 -358 266 333.7 8.56
Co 27 32 59 58.933 198 -66 802 -555 355 517.3 8.77
Zr 40 54 94 93.906 315 -93 685 -874 591 814.7 8.67
Ce 58 82 140 139.905 433 -94 567 -1 258 941 1 172.7 8.38
Ta 73 108 181 180.947 993 -52 007 -1 559 045 1 452.2 8.02
148 80 119 199 198.968 254 -31 746 -I 688 872 1 573.2 7.91
Th 90 142 232 232.038 051 38 051 -1 896 619 1 766.7 7.62
U 92 143 235 235.043 924 43 924 -1 915 060 1 783.9 7.59
U 92 144 236 236.045 563 45 563 -1 922 087 1 790.4 7.59
U 92 146 238 238.050 785 50 785 -I 934 195 1 801.7 7.57
PU 94 146 240 240.053 808 53 808 -1 946 821 1 813.5 7.56
~A = MA -- A (3.4)
Mass excess values are either given in u (or, more commonly, in micro mass units, #u) or
in eV (usually keV). Table 3.1 contains a number of atomic masses, mass excess, and mass
defect values, as well as some other information which is discussed in later sections.
When two dements form a compound in a chemical system, the amount of heat liberated
is a measure of the stability of the compound. The greater this heat of formation (enthalpy,
AH) the greater the stability of the compound. When carbon is combined with oxygen to
form CO 2, it is found experimentally that 393 kJ of heat is evolved per mole of CO 2
formed. If we use the Einstein relationship, we can calculate that this would correspond to
a total mass loss of 4.4 x 10 -9 g for each mole of CO 2 formed (44 g). Although chemists
do not doubt that this mass loss actually occurs, at present there are no instruments of
sufficient sensitivity to measure such small changes.
The energy changes in nuclear reactions are much larger. This can be seen if we use the
relationship between electron volts and joules (or calories) in Appendix IV, and observe that