Page 55 - Radiochemistry and nuclear chemistry
P. 55
44 Radiochemistry and Nuclear Chemistry
Positron emission and electron capture are competing processes with the probability of the
latter increasing as the atomic number increases. Beta decay is properly used to designate
all three processes, /3-,/3 +, and EC. (The term "beta decay" without any specification
usually only refers to/3- emission.)
Thus in the early part of the Periodic Table, unstable neutron deficient nuclides decay by
positron emission, but for the elements in the platinum region and beyond, decay occurs
predominantly by electron capture. Both processes are seen in isotopes of the elements in
the middle portion of the Periodic Table, see Figure 3.1 and Appendix C.
An alternative to positron decay (or EC) is proton emission, which, although rare, has
been observed in about 40 nuclei very far off the stability line. These nuclei all have
half-lives < 1 rain. For example: llSXe, t, h (p) 18 s; proton/EC ratio, 3 • 10 -3.
We can understand why the N/Z ratio must increase with atomic number in order to have
nuclear stability when we consider that the protons in the nucleus must experience a
repulsive Coulomb force. The fact that stable nuclei exist means that there must be an
attractive force tending to hold the neutrons and protons together. This attractive nuclear
force must be sufficient in stable nuclei to overcome the disruptive Coulomb force.
Conversely, in unstable nuclei there is a net imbalance between the attractive nuclear force
and the disruptive Coulomb force. As the number of protons increases, the total repulsive
Coulomb force must increase. Therefore, to provide sufficient attractive force for stability
the number of neutrons increases more rapidly than that of the protons.
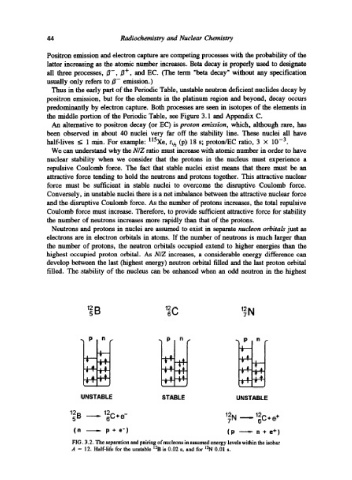
Neutrons and protons in nuclei are assumed to exist in separate nucleon orbitals just as
electrons are in electron orbitals in atoms. If the number of neutrons is much larger than
the number of protons, the neutron orbitals occupied extend to higher energies than the
highest occupied proton orbital. As N/Z increases, a considerable energy difference can
develop between the last (highest energy) neutron orbital filled and the last proton orbital
filled. The stability of the nucleus can be enhanced when an odd neutron in the highest
12 B 12 12N
6C
5
7
P "]Pin I
I
i=;
UNSTABLE STABLE UNSTABLE
12B5 ~ 12C+e- 12 N ~ 1~C§247
(n ----.- p + e') (p ---- n + e + )
FIG. 3.2. The separation and pairing of nucleons in assumed energy levels within the isobar
A = 12. Half-life for the unstable 12B is 0.02 s, and for 12N 0.01 s.