Page 75 - Radiochemistry and nuclear chemistry
P. 75
64 Radiochemistry and Nuclear Chemistry
a non-integral value, it violates the rule for conservation of angular momentum. Before
accounting for this discrepancy let us consider another aspect of/~-decay which seems
unusual.
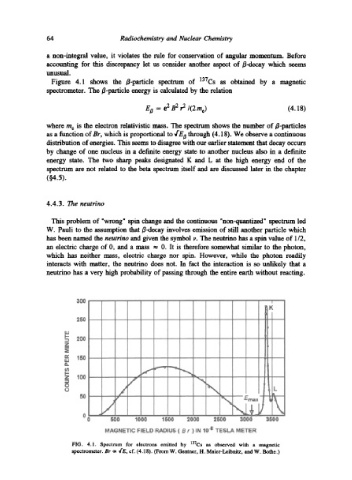
Figure 4.1 shows the /~-particle spectrum of 137Cs as obtained by a magnetic
spectrometer. The B-particle energy is calculated by the relation
EB = e 2 B 2 r 2/(2me) (4.18)
where m e is the electron relativistic mass. The spectrum shows the number of B-particles
as a function of Br, which is proportional to 4"EB through (4.18). We observe a continuous
distribution of energies. This seems to disagree with our earlier statement that decay occurs
by change of one nucleus in a definite energy state to another nucleus also in a definite
energy state. The two sharp peaks designated K and L at the high energy end of the
spectrum are not related to the beta spectrum itself and are discussed later in the chapter
(w
4.4.3. The neutrino
This problem of "wrong" spin change and the continuous "non-quantized" spectrum led
W. Pauli to the assumption that/3-decay involves emission of still another particle which
has been named the neutrino and given the symbol l,. The neutrino has a spin value of 1/2,
an electric charge of 0, and a mass ~ 0. It is therefore somewhat similar to the photon,
which has neither mass, electric charge nor spin. However, while the photon readily
interacts with matter, the neutrino does not. In fact the interaction is so unlikely that a
neutrino has a very high probability of passing through the entire earth without reacting.
FIG. 4.1. Spectrum for electrons emitted by 137Cs as observed with a magnetic
spectrometer. 8r oc ,['E, cf. (4.18). (From W. Gentner, H. Maier-Leibnitz, and W. Bothe.)