Page 344 - Reservoir Formation Damage
P. 344
324 Reservoir Formation Damage
temperature during the production of reservoir fluids. Scaling associated
with the improved recovery processes, such as water, carbonated water,
alkaline water, and carbon dioxide injection, may be caused by mixing
incompatible fluids and/or pressure and temperature variations.
The scale formation mechanisms can be classified as: (1) natural
scaling and (2) induced scaling (Amaefule et al., 1988). These mechanisms
can be best explained by means of scale precipitation charts, such as those
given by Shaughnessy and Kline (1982, 1983), who developed practical
+2
charts depicting the relationships between dissolved calcium (Ca ) and
bicarbonate (HCO^ ions, calcium carbonate (CaCO 3) precipitate, CO 2
partial and total pressures, and temperature, based on the equilibrium
relationship for the calcium carbonate scale formation by the reaction
+2
Ca CaCO 3(s) + CO 2(g} + H 2O (13-1)
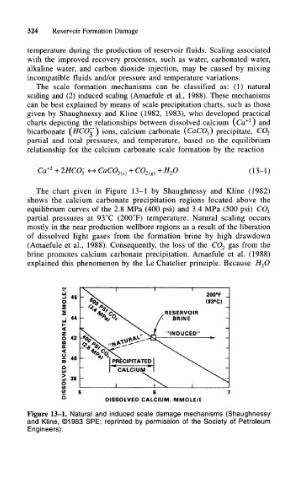
The chart given in Figure 13-1 by Shaughnessy and Kline (1982)
shows the calcium carbonate precipitation regions located above the
equilibrium curves of the 2.8 MPa (400 psi) and 3.4 MPa (500 psi) CO 2
partial pressures at 93°C (200°F) temperature. Natural scaling occurs
mostly in the near production wellbore regions as a result of the liberation
of dissolved light gases from the formation brine by high drawdown
(Amaefule et al., 1988). Consequently, the loss of the CO 2 gas from the
brine promotes calcium carbonate precipitation. Amaefule et al. (1988)
explained this phenomenon by the Le Chatelier principle. Because H 2O
200«F
46
C)
44
42
~ 40
Q
01
3 38
O
V)
<2 s
DISSOLVED CALCIUM, MMOLE/B
Figure 13-1. Natural and induced scale damage mechanisms (Shaughnessy
and Kline, ©1983 SPE; reprinted by permission of the Society of Petroleum
Engineers).