Page 250 - Schaum's Outline of Theory and Problems of Applied Physics
P. 250
CHAP. 20] KINETIC THEORY OF MATTER 235
Here T = 100 C = 373 K, and so
◦
3kT (3)(1.38 × 10 −23 J/K)(373 K)
4
v av = = = 29.1 × 10 m/s
m 5.3 × 10 −26 kg
2
= 5.4 × 10 m/s = 540 m/s
SOLVED PROBLEM 20.5
◦
A certain tank holds 1 g of hydrogen at 0 C, and another identical tank holds1gof oxygen at 0 C. The
◦
mass of an oxygen molecule is 16 times greater than that of a hydrogen molecule. (a) Which tank contains
more molecules? How many more? (b) Which gas exerts the greater pressure? How much greater? (c) In
which gas do the molecules have greater average energies? How much greater? (d) In which gas do the
molecules have greater average velocities? How much greater?
(a) There are 16 times more hydrogen molecules.
(b) The hydrogen pressure is 16 times greater because there are 16 times more molecules to exert force on the
container walls.
(c) The average molecular energies are the same in both gases because their temperatures are the same.
√
(d) The average velocity of the hydrogen molecules is 16 = 4 times more than that of the oxygen molecules
√
because the hydrogen molecules are 16 times lighter and v av = 3kT/m.
RELATIVE HUMIDITY
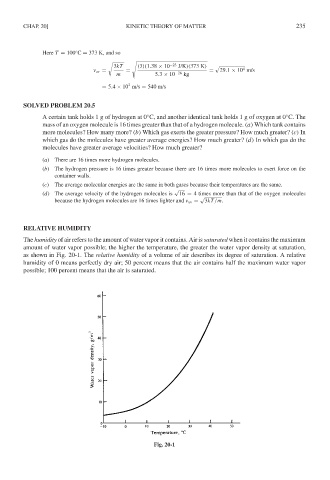
The humidity of air refers to the amount of water vapor it contains. Air is saturated when it contains the maximum
amount of water vapor possible; the higher the temperature, the greater the water vapor density at saturation,
as shown in Fig. 20-1. The relative humidity of a volume of air describes its degree of saturation. A relative
humidity of 0 means perfectly dry air; 50 percent means that the air contains half the maximum water vapor
possible; 100 percent means that the air is saturated.
Fig. 20-1