Page 450 - Schaum's Outline of Theory and Problems of Applied Physics
P. 450
CHAP. 35] THE SOLID STATE 435
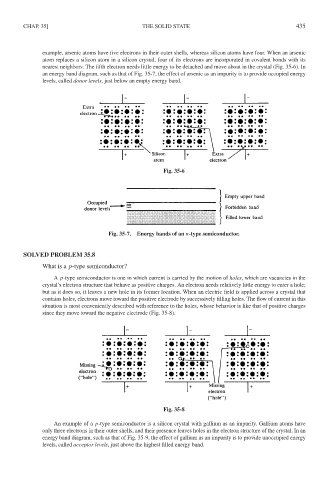
example, arsenic atoms have five electrons in their outer shells, whereas silicon atoms have four. When an arsenic
atom replaces a silicon atom in a silicon crystal, four of its electrons are incorporated in covalent bonds with its
nearest neighbors. The fifth electron needs little energy to be detached and move about in the crystal (Fig. 35-6). In
an energy band diagram, such as that of Fig. 35-7, the effect of arsenic as an impurity is to provide occupied energy
levels, called donor levels, just below an empty energy band.
Fig. 35-6
Fig. 35-7. Energy bands of an n-type semiconductor.
SOLVED PROBLEM 35.8
What is a p-type semiconductor?
A p-type semiconductor is one in which current is carried by the motion of holes, which are vacancies in the
crystal’s electron structure that behave as positive charges. An electron needs relatively little energy to enter a hole;
but as it does so, it leaves a new hole in its former location. When an electric field is applied across a crystal that
contains holes, electrons move toward the positive electrode by successively filling holes. The flow of current in this
situation is most conveniently described with reference to the holes, whose behavior is like that of positive charges
since they move toward the negative electrode (Fig. 35-8).
Fig. 35-8
An example of a p-type semiconductor is a silicon crystal with gallium as an impurity. Gallium atoms have
only three electrons in their outer shells, and their presence leaves holes in the electron structure of the crystal. In an
energy band diagram, such as that of Fig. 35-9, the effect of gallium as an impurity is to provide unoccupied energy
levels, called acceptor levels, just above the highest filled energy band.