Page 459 - Schaum's Outline of Theory and Problems of Applied Physics
P. 459
444 NUCLEAR PHYSICS [CHAP. 36
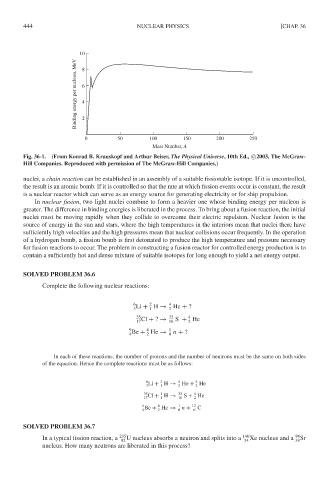
10 8
Binding energy per nucleon, MeV 6 4
2
0 50 100 150 200 250
Mass Number, A
Fig. 36-1. (From Konrad B. Krauskopf and Arthur Beiser, The Physical Universe, 10th Ed., c 2003, The McGraw-
Hill Companies. Reproduced with permission of The McGraw-Hill Companies.)
nuclei, a chain reaction can be established in an assembly of a suitable fissionable isotope. If it is uncontrolled,
the result is an atomic bomb. If it is controlled so that the rate at which fission events occur is constant, the result
is a nuclear reactor which can serve as an energy source for generating electricity or for ship propulsion.
In nuclear fusion, two light nuclei combine to form a heavier one whose binding energy per nucleon is
greater. The difference in binding energies is liberated in the process. To bring about a fusion reaction, the initial
nuclei must be moving rapidly when they collide to overcome their electric repulsion. Nuclear fusion is the
source of energy in the sun and stars, where the high temperatures in the interiors mean that nuclei there have
sufficiently high velocities and the high pressures mean that nuclear collisions occur frequently. In the operation
of a hydrogen bomb, a fission bomb is first detonated to produce the high temperature and pressure necessary
for fusion reactions to occur. The problem in constructing a fusion reactor for controlled energy production is to
contain a sufficiently hot and dense mixture of suitable isotopes for long enough to yield a net energy output.
SOLVED PROBLEM 36.6
Complete the following nuclear reactions:
6 2 4
3 Li + H → He + ?
1
2
35 32 4
17 Cl + ? → 16 S + He
2
9 4 1
2
0
4 Be + He → n + ?
In each of these reactions, the number of protons and the number of neutrons must be the same on both sides
of the equation. Hence the complete reactions must be as follows:
4
2
4
6 Li + H → He + He
3 1 2 2
35 1 32 S + He
4
1
17 Cl + H → 16 2
9 4 1 12 C
0
4 Be + He → n + 6
2
SOLVED PROBLEM 36.7
In a typical fission reaction, a 235 U nucleus absorbs a neutron and splits into a 140 Xe nucleus and a 94 Sr
92 54 38
nucleus. How many neutrons are liberated in this process?