Page 188 - Theory and Problems of BEGINNING CHEMISTRY
P. 188
CHAP. 12] GASES 177
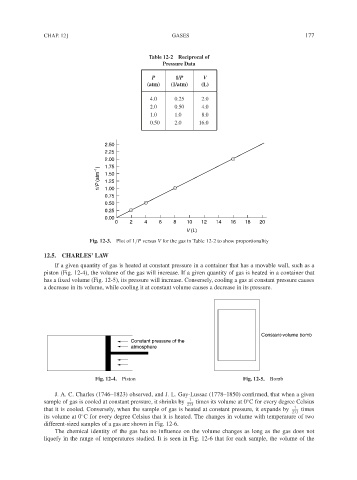
Table 12-2 Reciprocal of
Pressure Data
P 1/P V
(atm) (1/atm) (L)
4.0 0.25 2.0
2.0 0.50 4.0
1.0 1.0 8.0
0.50 2.0 16.0
2.50
2.25
2.00
1.75
1/P (atm −1 ) 1.50
1.25
1.00
0.75
0.50
0.25
0.00
0 2 4 6 8 10 12 14 16 18 20
V (L)
Fig. 12-3. Plot of 1/P versus V for the gas in Table 12-2 to show proportionality
12.5. CHARLES’ LAW
If a given quantity of gas is heated at constant pressure in a container that has a movable wall, such as a
piston (Fig. 12-4), the volume of the gas will increase. If a given quantity of gas is heated in a container that
has a fixed volume (Fig. 12-5), its pressure will increase. Conversely, cooling a gas at constant pressure causes
a decrease in its volume, while cooling it at constant volume causes a decrease in its pressure.
Constant-volume bomb
Constant pressure of the
atmosphere
Fig. 12-4. Piston Fig. 12-5. Bomb
J. A. C. Charles (1746–1823) observed, and J. L. Gay-Lussac (1778–1850) confirmed, that when a given
sample of gas is cooled at constant pressure, it shrinks by 1 times its volume at 0 C for every degree Celsius
◦
273
that it is cooled. Conversely, when the sample of gas is heated at constant pressure, it expands by 1 times
273
its volume at 0 C for every degree Celsius that it is heated. The changes in volume with temperature of two
◦
different-sized samples of a gas are shown in Fig. 12-6.
The chemical identity of the gas has no influence on the volume changes as long as the gas does not
liquefy in the range of temperatures studied. It is seen in Fig. 12-6 that for each sample, the volume of the