Page 98 - Science at the nanoscale
P. 98
7:6
RPS: PSP0007 - Science-at-Nanoscale
June 12, 2009
From Atoms and Molecules to Nanoscale Materials
88
and the rigidity needed of the complex structures. This brings
us to the fascinating research field of supramolecules and self-
assembly processes.
Supramolecules
4.4.2
Historically, chemists started off by developing synthetic method-
ologies that form strong bonding such as the C–C, C–O, etc.
covalent bonds. In supramolecular synthesis, attention is focused
on the weaker and reversible non-covalent interactions such as hy-
drogen bonding, van der Waals forces, π–π interaction, metal co-
ordination, etc. These syntheses require careful design of the mo-
tif (molecules or segments of a molecule) such that it contains the
necessary functionalities that will allow it to integrate to form a
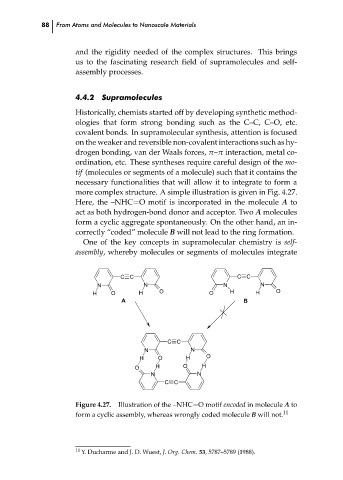
more complex structure. A simple illustration is given in Fig. 4.27.
Here, the –NHC=O motif is incorporated in the molecule A to
act as both hydrogen-bond donor and acceptor. Two A molecules
form a cyclic aggregate spontaneously. On the other hand, an in-
correctly “coded” molecule B will not lead to the ring formation.
One of the key concepts in supramolecular chemistry is self-
assembly, whereby molecules or segments of molecules integrate
C
C
N
N
O
H
O
H
A
C C O N H C B C H N O ch04
N
N
O
H O H
H
H O
O
N N
C C
Figure 4.27. Illustration of the –NHC=O motif encoded in molecule A to
form a cyclic assembly, whereas wrongly coded molecule B will not. 11
11 Y. Ducharme and J. D. Wuest, J. Org. Chem. 53, 5787–5789 (1988).