Page 96 - Science at the nanoscale
P. 96
7:6
RPS: PSP0007 - Science-at-Nanoscale
June 12, 2009
From Atoms and Molecules to Nanoscale Materials
86
a
Counts(a.u.)
b
(111)
(220)
(311)
c
60
20
80
40
2θ/degree
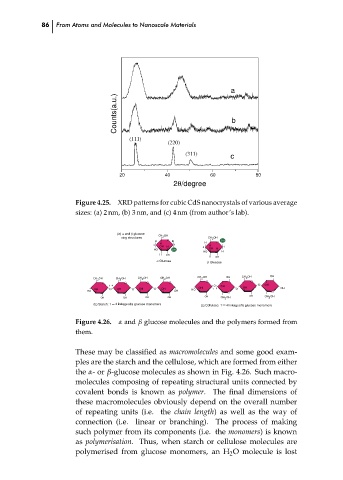
Figure 4.25.
XRD patterns for cubic CdS nanocrystals of various average
sizes: (a) 2 nm, (b) 3 nm, and (c) 4 nm (from author’s lab).
(a) α and β glucose
CH 2 OH
CH 2 OH
ring structures
O
OH
H
H
O
H
H
1
4
H
OH
H
1
4
OH
H
HO
OH
H
HO
H
OH
H
OH
α Glucose
β Glucose
CH 2 OH
CH 2 OH
CH 2 OH
CH 2 OH
CH 2 OH
OH
CH 2 OH
OH
O
O
O
O
O
O
O
O
1 4
OH
OH
OH
OH
1 4
OH
OH
O
O
OH
OH
O
O
HO
OH
HO
OH
O
O
OH
CH 2 OH
CH 2 OH
OH
OH
OH
OH
OH
4 linkageof a glucose monomers
(b) Starch: 1
(c) Cellulose: 1
4 linkageof b glucose monomers
α and β glucose molecules and the polymers formed from
Figure 4.26.
them.
These may be classified as macromolecules and some good exam- ch04
ples are the starch and the cellulose, which are formed from either
the α- or β-glucose molecules as shown in Fig. 4.26. Such macro-
molecules composing of repeating structural units connected by
covalent bonds is known as polymer. The final dimensions of
these macromolecules obviously depend on the overall number
of repeating units (i.e. the chain length) as well as the way of
connection (i.e. linear or branching). The process of making
such polymer from its components (i.e. the monomers) is known
as polymerisation. Thus, when starch or cellulose molecules are
polymerised from glucose monomers, an H 2 O molecule is lost