Page 92 - Science at the nanoscale
P. 92
7:6
RPS: PSP0007 - Science-at-Nanoscale
June 12, 2009
From Atoms and Molecules to Nanoscale Materials
82
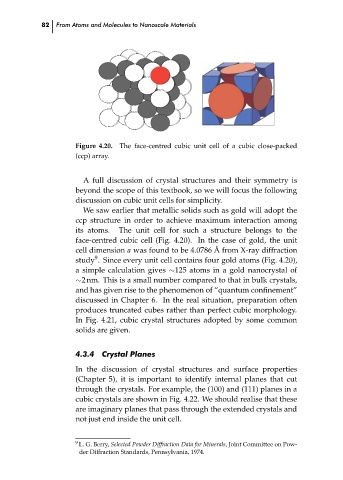
The face-centred cubic unit cell of a cubic close-packed
Figure 4.20.
(ccp) array.
A full discussion of crystal structures and their symmetry is
beyond the scope of this textbook, so we will focus the following
discussion on cubic unit cells for simplicity.
We saw earlier that metallic solids such as gold will adopt the
ccp structure in order to achieve maximum interaction among
its atoms.
The unit cell for such a structure belongs to the
face-centred cubic cell (Fig. 4.20). In the case of gold, the unit
cell dimension a was found to be 4.0786 ˚ A from X-ray diffraction
study . Since every unit cell contains four gold atoms (Fig. 4.20),
a simple calculation gives ∼125 atoms in a gold nanocrystal of
∼2 nm. This is a small number compared to that in bulk crystals,
and has given rise to the phenomenon of “quantum confinement”
discussed in Chapter 6. In the real situation, preparation often
produces truncated cubes rather than perfect cubic morphology.
In Fig. 4.21, cubic crystal structures adopted by some common
solids are given.
4.3.4 9 Crystal Planes ch04
In the discussion of crystal structures and surface properties
(Chapter 5), it is important to identify internal planes that cut
through the crystals. For example, the (100) and (111) planes in a
cubic crystals are shown in Fig. 4.22. We should realise that these
are imaginary planes that pass through the extended crystals and
not just end inside the unit cell.
9 L. G. Berry, Selected Powder Diffraction Data for Minerals, Joint Committee on Pow-
der Diffraction Standards, Pennsylvania, 1974.