Page 87 - Science at the nanoscale
P. 87
7:6
RPS: PSP0007 - Science-at-Nanoscale
June 12, 2009
4.3. From Atoms to Solid Materials
H
A
T
N
O
H
N
C
G
A
T
N
N
H
N
C
G
Sugar Phosphate
N
N
G
C
O
Adenine
A
Thymine
T
C
G
Sugar Phosphate
H
A
T
O
H
N
N
A
T
G
C
N
N
H
N
T
A
N
N
G
C
Sugar Phosphate
O
H
N
C
G
H
Cytosine
Guanine
A
T
G
C
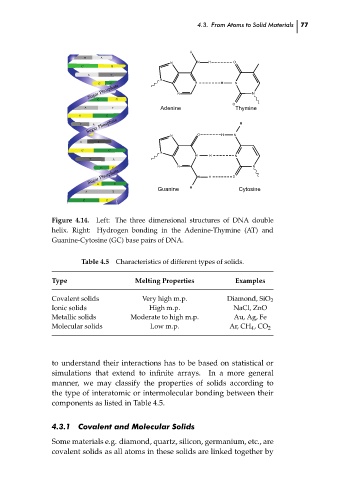
Figure 4.14.
Left: The three dimensional structures of DNA double
helix. Right: Hydrogen bonding in the Adenine-Thymine (AT) and
Guanine-Cytosine (GC) base pairs of DNA.
Table 4.5 Characteristics of different types of solids.
Type
Examples
Melting Properties
Very high m.p.
Covalent solids
Diamond, SiO 2
High m.p.
Ionic solids
NaCl, ZnO
Moderate to high m.p.
Au, Ag, Fe
Metallic solids
Molecular solids
Low m.p.
Ar, CH 4 , CO 2
to understand their interactions has to be based on statistical or 77 ch04
simulations that extend to infinite arrays. In a more general
manner, we may classify the properties of solids according to
the type of interatomic or intermolecular bonding between their
components as listed in Table 4.5.
4.3.1 Covalent and Molecular Solids
Some materials e.g. diamond, quartz, silicon, germanium, etc., are
covalent solids as all atoms in these solids are linked together by