Page 85 - Science at the nanoscale
P. 85
7:6
RPS: PSP0007 - Science-at-Nanoscale
June 12, 2009
4.2. Molecules and Molecular Interactions
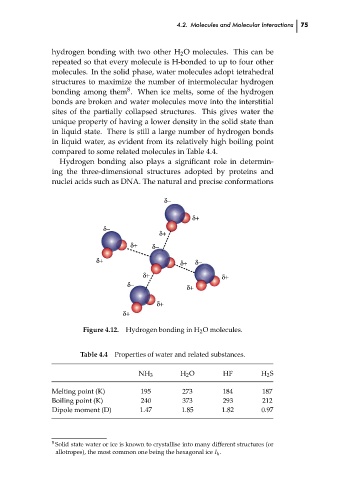
hydrogen bonding with two other H 2 O molecules. This can be
repeated so that every molecule is H-bonded to up to four other
molecules. In the solid phase, water molecules adopt tetrahedral
structures to maximize the number of intermolecular hydrogen
8
bonding among them . When ice melts, some of the hydrogen
bonds are broken and water molecules move into the interstitial
sites of the partially collapsed structures. This gives water the
unique property of having a lower density in the solid state than
in liquid state. There is still a large number of hydrogen bonds
in liquid water, as evident from its relatively high boiling point
compared to some related molecules in Table 4.4.
Hydrogen bonding also plays a significant role in determin-
ing the three-dimensional structures adopted by proteins and
nuclei acids such as DNA. The natural and precise conformations
δ−
δ+
δ−
δ+
δ+
δ−
δ+
δ−
δ+
δ+
δ+
δ−
δ+
δ+
δ+
Hydrogen bonding in H 2 O molecules.
Figure 4.12.
Table 4.4 Properties of water and related substances. 75 ch04
NH 3 H 2 O HF H 2 S
Melting point (K) 195 273 184 187
Boiling point (K) 240 373 293 212
Dipole moment (D) 1.47 1.85 1.82 0.97
8 Solid state water or ice is known to crystallise into many different structures (or
allotropes), the most common one being the hexagonal ice I h .