Page 81 - Science at the nanoscale
P. 81
RPS: PSP0007 - Science-at-Nanoscale
7:6
June 12, 2009
4.2. Molecules and Molecular Interactions
+
+
+
Maximum attraction
Orientation dependent
Maximum repulsion
interaction
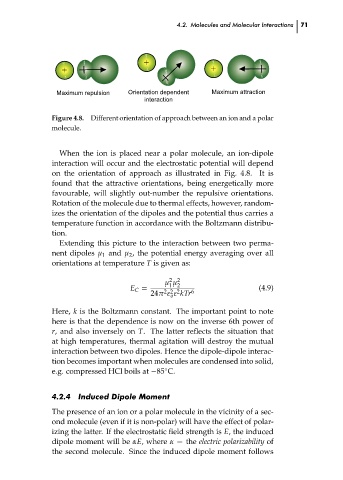
Different orientation of approach between an ion and a polar
Figure 4.8.
molecule.
When the ion is placed near a polar molecule, an ion-dipole
interaction will occur and the electrostatic potential will depend
on the orientation of approach as illustrated in Fig. 4.8. It is
found that the attractive orientations, being energetically more
favourable, will slightly out-number the repulsive orientations.
Rotation of the molecule due to thermal effects, however, random-
izes the orientation of the dipoles and the potential thus carries a
temperature function in accordance with the Boltzmann distribu-
tion.
Extending this picture to the interaction between two perma-
nent dipoles µ 1 and µ 2 , the potential energy averaging over all
orientations at temperature T is given as:
2 2
µ µ
1 2
(4.9)
E C =
2 2 2
6
24π ε ε kTr
o
Here, k is the Boltzmann constant. The important point to note
here is that the dependence is now on the inverse 6th power of
r, and also inversely on T. The latter reflects the situation that
at high temperatures, thermal agitation will destroy the mutual 71 ch04
interaction between two dipoles. Hence the dipole-dipole interac-
tion becomes important when molecules are condensed into solid,
e.g. compressed HCl boils at −85 C.
◦
4.2.4 Induced Dipole Moment
The presence of an ion or a polar molecule in the vicinity of a sec-
ond molecule (even if it is non-polar) will have the effect of polar-
izing the latter. If the electrostatic field strength is E, the induced
dipole moment will be αE, where α = the electric polarizability of
the second molecule. Since the induced dipole moment follows