Page 77 - Science at the nanoscale
P. 77
7:6
RPS: PSP0007 - Science-at-Nanoscale
June 12, 2009
4.2. Molecules and Molecular Interactions
*
(2p)
σ
*(2p)
π
2p
2p
(2p)
σ
(2p)
π
*
(2s)
σ
2s
2s
(2s)
σ
*(1s)
σ
1s
1s
N atom
N atom
(1s)
σ
N 2
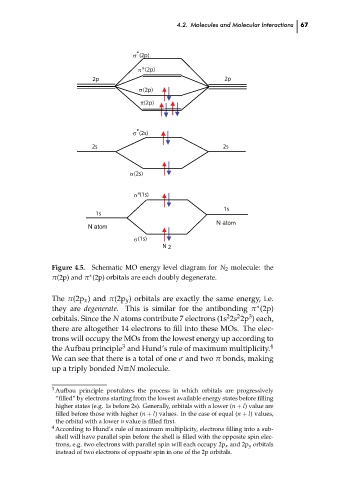
Figure 4.5. Schematic MO energy level diagram for N 2 molecule: the
π(2p) and π (2p) orbitals are each doubly degenerate.
∗
The π(2p x ) and π(2p y ) orbitals are exactly the same energy, i.e.
∗
they are degenerate. This is similar for the antibonding π (2p)
2
2
3
orbitals. Since the N atoms contribute 7 electrons (1s 2s 2p ) each,
there are altogether 14 electrons to fill into these MOs. The elec-
trons will occupy the MOs from the lowest energy up according to 67 ch04
3
the Aufbau principle and Hund’s rule of maximum multiplicity. 4
We can see that there is a total of one σ and two π bonds, making
up a triply bonded N≡N molecule.
3 Aufbau principle postulates the process in which orbitals are progressively
“filled” by electrons starting from the lowest available energy states before filling
higher states (e.g. 1s before 2s). Generally, orbitals with a lower (n + l) value are
filled before those with higher (n + l) values. In the case of equal (n + l) values,
the orbital with a lower n value is filled first.
4 According to Hund’s rule of maximum multiplicity, electrons filling into a sub-
shell will have parallel spin before the shell is filled with the opposite spin elec-
trons, e.g. two electrons with parallel spin will each occupy 2p x and 2p y orbitals
instead of two electrons of opposite spin in one of the 2p orbitals.