Page 75 - Science at the nanoscale
P. 75
RPS: PSP0007 - Science-at-Nanoscale
7:6
June 12, 2009
4.2. Molecules and Molecular Interactions
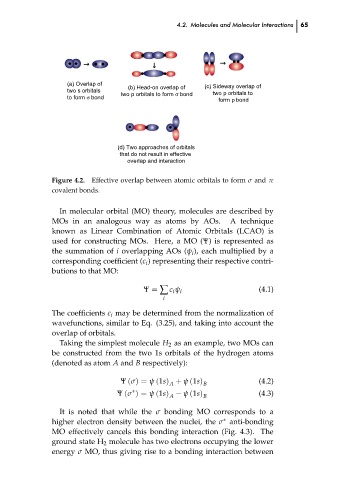
(a) Overlap of
(b) Head-on overlap of
two s orbitals
two p orbitals to
two p orbitals to form σ bond
to form σ bond
form p bond
that do not result in effective
overlap and interaction
Figure 4.2. Effective overlap between atomic orbitals to form σ and π
covalent bonds.
In molecular orbital (MO) theory, molecules are described by
A technique
MOs in an analogous way as atoms by AOs.
known as Linear Combination of Atomic Orbitals (LCAO) is
used for constructing MOs. Here, a MO (Ψ) is represented as
the summation of i overlapping AOs (ψ ), each multiplied by a
corresponding coefficient (c ) representing their respective contri-
i
butions to that MO:
c
∑ i ψ i
Ψ =
i
The coefficients c may be determined from the normalization of
i
wavefunctions, similar to Eq. (3.25), and taking into account the
overlap of orbitals. (d) Two approaches of orbitals i (c) Sideway overlap of (4.1) 65 ch04
Taking the simplest molecule H 2 as an example, two MOs can
be constructed from the two 1s orbitals of the hydrogen atoms
(denoted as atom A and B respectively):
Ψ (σ) = ψ (1s) + ψ (1s) B (4.2)
A
∗
Ψ (σ ) = ψ (1s) − ψ (1s) B (4.3)
A
It is noted that while the σ bonding MO corresponds to a
higher electron density between the nuclei, the σ anti-bonding
∗
MO effectively cancels this bonding interaction (Fig. 4.3). The
ground state H 2 molecule has two electrons occupying the lower
energy σ MO, thus giving rise to a bonding interaction between