Page 79 - Science at the nanoscale
P. 79
7:6
RPS: PSP0007 - Science-at-Nanoscale
June 12, 2009
4.2. Molecules and Molecular Interactions
we say that the Cl atom is more electronegative than the H atom
and that the HCl molecule is polar. In the MOs constructed, the
respective coefficients (c i in Eq. 4.1) will reflect the uneven contri-
butions of the respective AOs. For example, the bonding orbital
5
of HCl may be empirically written as :
(4.6)
Ψ (σ) = 0.57ψ (1s) + 0.73ψ (2p)
H
Cl
Since the electron probability density is given by the square of
the wave function coefficient, we estimate that the bonding elec-
2
2
2
trons spend ∼ 0.73 ÷ (0.57 + 0.73 ) = 62% of their time at the
Cl atom. For the extreme situation when the bonding electrons
are distributed ∼100% over one atom rather than the other, the
molecule may be more appropriately described as A B . The
+ −
ionic bond thus formed may then be ascribed to the Coulombic
force of attraction between the two ions.
4.2.2
Dipole Moment
Classically, two equal but opposite charges +δ and −δ separated
by a distance l produce a dipole moment µ given as:
µ = δ × l
(4.7)
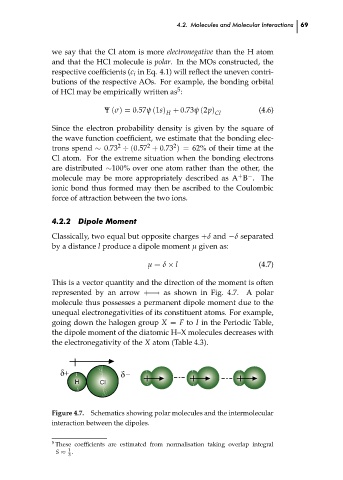
This is a vector quantity and the direction of the moment is often
represented by an arrow +−→ as shown in Fig. 4.7. A polar
molecule thus possesses a permanent dipole moment due to the
unequal electronegativities of its constituent atoms. For example,
going down the halogen group X = F to I in the Periodic Table,
the dipole moment of the diatomic H–X molecules decreases with 69 ch04
the electronegativity of the X atom (Table 4.3).
į+ į _
H Cl
Figure 4.7. Schematics showing polar molecules and the intermolecular
interaction between the dipoles.
5 These coefficients are estimated from normalisation taking overlap integral
1
S ≈ .
3