Page 83 - Science at the nanoscale
P. 83
RPS: PSP0007 - Science-at-Nanoscale
7:6
June 12, 2009
4.2. Molecules and Molecular Interactions
energy
Interaction 0
Intermolecular distance
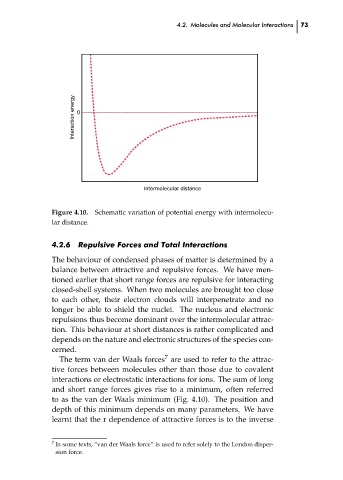
Schematic variation of potential energy with intermolecu-
Figure 4.10.
lar distance.
Repulsive Forces and Total Interactions
4.2.6
The behaviour of condensed phases of matter is determined by a
balance between attractive and repulsive forces. We have men-
tioned earlier that short range forces are repulsive for interacting
closed-shell systems. When two molecules are brought too close
to each other, their electron clouds will interpenetrate and no
longer be able to shield the nuclei. The nucleus and electronic
repulsions thus become dominant over the intermolecular attrac-
tion. This behaviour at short distances is rather complicated and
depends on the nature and electronic structures of the species con- 73 ch04
cerned.
7
The term van der Waals forces are used to refer to the attrac-
tive forces between molecules other than those due to covalent
interactions or electrostatic interactions for ions. The sum of long
and short range forces gives rise to a minimum, often referred
to as the van der Waals minimum (Fig. 4.10). The position and
depth of this minimum depends on many parameters. We have
learnt that the r dependence of attractive forces is to the inverse
7 In some texts, “van der Waals force” is used to refer solely to the London disper-
sion force.