Page 86 - Science at the nanoscale
P. 86
7:6
June 12, 2009
From Atoms and Molecules to Nanoscale Materials
76
O
H
O
R
N
N
N
... H
... H
O
R
...
O
R
H
O
N
N
N
H
H
O
R
beta-pleated sheets
alpha helix
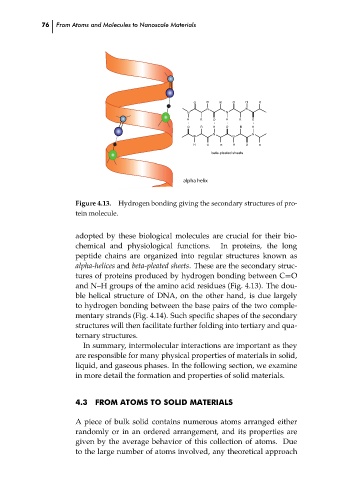
Figure 4.13.
Hydrogen bonding giving the secondary structures of pro-
tein molecule.
adopted by these biological molecules are crucial for their bio-
In proteins, the long
chemical and physiological functions.
peptide chains are organized into regular structures known as
alpha-helices and beta-pleated sheets. These are the secondary struc-
tures of proteins produced by hydrogen bonding between C=O
and N–H groups of the amino acid residues (Fig. 4.13). The dou-
ble helical structure of DNA, on the other hand, is due largely
to hydrogen bonding between the base pairs of the two comple-
mentary strands (Fig. 4.14). Such specific shapes of the secondary
structures will then facilitate further folding into tertiary and qua-
ternary structures. RPS: PSP0007 - Science-at-Nanoscale R R H N O O H N ... R R ch04
In summary, intermolecular interactions are important as they
are responsible for many physical properties of materials in solid,
liquid, and gaseous phases. In the following section, we examine
in more detail the formation and properties of solid materials.
4.3 FROM ATOMS TO SOLID MATERIALS
A piece of bulk solid contains numerous atoms arranged either
randomly or in an ordered arrangement, and its properties are
given by the average behavior of this collection of atoms. Due
to the large number of atoms involved, any theoretical approach