Page 88 - Science at the nanoscale
P. 88
7:6
RPS: PSP0007 - Science-at-Nanoscale
June 12, 2009
From Atoms and Molecules to Nanoscale Materials
78
(a)
(b)
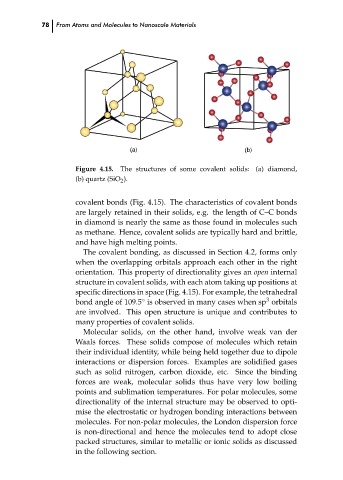
Figure 4.15.
The structures of some covalent solids: (a) diamond,
(b) quartz (SiO 2 ).
covalent bonds (Fig. 4.15). The characteristics of covalent bonds
are largely retained in their solids, e.g. the length of C–C bonds
in diamond is nearly the same as those found in molecules such
as methane. Hence, covalent solids are typically hard and brittle,
and have high melting points.
The covalent bonding, as discussed in Section 4.2, forms only
when the overlapping orbitals approach each other in the right
orientation. This property of directionality gives an open internal
structure in covalent solids, with each atom taking up positions at
specific directions in space (Fig. 4.15). For example, the tetrahedral
3
◦
bond angle of 109.5 is observed in many cases when sp orbitals
are involved. This open structure is unique and contributes to
many properties of covalent solids.
Molecular solids, on the other hand, involve weak van der
Waals forces. These solids compose of molecules which retain ch04
their individual identity, while being held together due to dipole
interactions or dispersion forces. Examples are solidified gases
such as solid nitrogen, carbon dioxide, etc. Since the binding
forces are weak, molecular solids thus have very low boiling
points and sublimation temperatures. For polar molecules, some
directionality of the internal structure may be observed to opti-
mise the electrostatic or hydrogen bonding interactions between
molecules. For non-polar molecules, the London dispersion force
is non-directional and hence the molecules tend to adopt close
packed structures, similar to metallic or ionic solids as discussed
in the following section.