Page 90 - Science at the nanoscale
P. 90
7:6
RPS: PSP0007 - Science-at-Nanoscale
June 12, 2009
From Atoms and Molecules to Nanoscale Materials
80
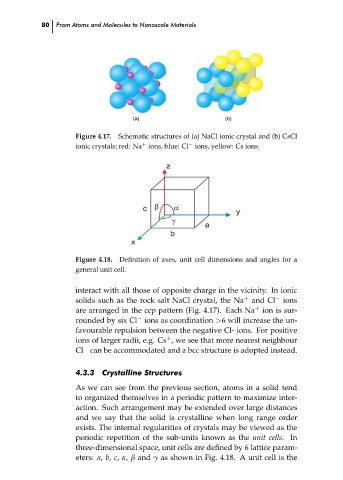
(a)
(b)
Schematic structures of (a) NaCl ionic crystal and (b) CsCl
Figure 4.17.
ionic crystals; red: Na ions, blue: Cl ions, yellow: Cs ions.
−
+
Figure 4.18.
Definition of axes, unit cell dimensions and angles for a
general unit cell.
interact with all those of opposite charge in the vicinity. In ionic
ions
−
and Cl
solids such as the rock salt NaCl crystal, the Na
+
are arranged in the ccp pattern (Fig. 4.17). Each Na ion is sur-
+
−
rounded by six Cl ions as coordination >6 will increase the un-
favourable repulsion between the negative Cl- ions. For positive
ions of larger radii, e.g. Cs , we see that more nearest neighbour ch04
+
Cl can be accommodated and a bcc structure is adopted instead.
−
4.3.3 Crystalline Structures
As we can see from the previous section, atoms in a solid tend
to organized themselves in a periodic pattern to maximize inter-
action. Such arrangement may be extended over large distances
and we say that the solid is crystalline when long range order
exists. The internal regularities of crystals may be viewed as the
periodic repetition of the sub-units known as the unit cells. In
three-dimensional space, unit cells are defined by 6 lattice param-
eters: a, b, c, α, β and γ as shown in Fig. 4.18. A unit cell is the