Page 82 - Science at the nanoscale
P. 82
7:6
RPS: PSP0007 - Science-at-Nanoscale
June 12, 2009
From Atoms and Molecules to Nanoscale Materials
72
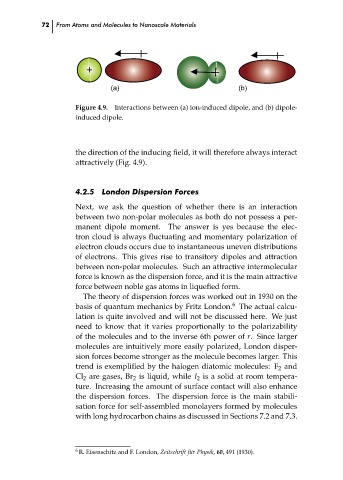
+
(a)
(b)
Figure 4.9. Interactions between (a) ion-induced dipole, and (b) dipole-
induced dipole.
the direction of the inducing field, it will therefore always interact
attractively (Fig. 4.9).
4.2.5
London Dispersion Forces
Next, we ask the question of whether there is an interaction
between two non-polar molecules as both do not possess a per-
The answer is yes because the elec-
manent dipole moment.
tron cloud is always fluctuating and momentary polarization of
electron clouds occurs due to instantaneous uneven distributions
of electrons. This gives rise to transitory dipoles and attraction
between non-polar molecules. Such an attractive intermolecular
force is known as the dispersion force, and it is the main attractive
force between noble gas atoms in liquefied form.
The theory of dispersion forces was worked out in 1930 on the
6
basis of quantum mechanics by Fritz London. The actual calcu-
lation is quite involved and will not be discussed here. We just
need to know that it varies proportionally to the polarizability
of the molecules and to the inverse 6th power of r. Since larger ch04
molecules are intuitively more easily polarized, London disper-
sion forces become stronger as the molecule becomes larger. This
trend is exemplified by the halogen diatomic molecules: F 2 and
Cl 2 are gases, Br 2 is liquid, while I 2 is a solid at room tempera-
ture. Increasing the amount of surface contact will also enhance
the dispersion forces. The dispersion force is the main stabili-
sation force for self-assembled monolayers formed by molecules
with long hydrocarbon chains as discussed in Sections 7.2 and 7.3.
6 R. Eisenschitz and F. London, Zeitschrift f¨ur Physik, 60, 491 (1930).