Page 104 - Sedimentology and Stratigraphy
P. 104
Weathering Processes 91
1 2
/$
$
0 %
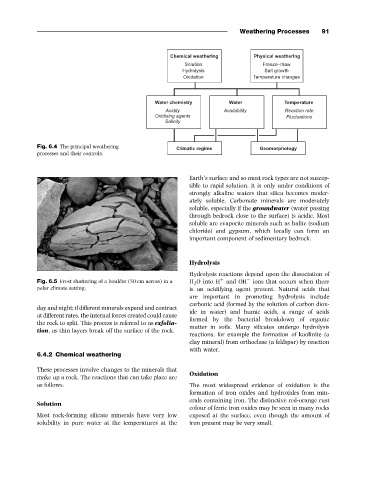
Fig. 6.4 The principal weathering
processes and their controls.
Earth’s surface and so most rock types are not suscep-
tible to rapid solution. It is only under conditions of
strongly alkaline waters that silica becomes moder-
ately soluble. Carbonate minerals are moderately
soluble, especially if the groundwater (water passing
through bedrock close to the surface) is acidic. Most
soluble are evaporite minerals such as halite (sodium
chloride) and gypsum, which locally can form an
important component of sedimentary bedrock.
Hydrolysis
Hydrolysis reactions depend upon the dissociation of
þ
Fig. 6.5 Frost shattering of a boulder (50 cm across) in a H 2 O into H and OH ions that occurs when there
polar climate setting. is an acidifying agent present. Natural acids that
are important in promoting hydrolysis include
carbonic acid (formed by the solution of carbon diox-
day and night; if different minerals expand and contract
ide in water) and humic acids, a range of acids
at different rates, the internal forces created could cause
formed by the bacterial breakdown of organic
the rock to split. This process is referred to as exfolia-
matter in soils. Many silicates undergo hydrolysis
tion, as thin layers break off the surface of the rock.
reactions, for example the formation of kaolinite (a
clay mineral) from orthoclase (a feldspar) by reaction
with water.
6.4.2 Chemical weathering
These processes involve changes to the minerals that
Oxidation
make up a rock. The reactions that can take place are
as follows. The most widespread evidence of oxidation is the
formation of iron oxides and hydroxides from min-
erals containing iron. The distinctive red-orange rust
Solution
colour of ferric iron oxides may be seen in many rocks
Most rock-forming silicate minerals have very low exposed at the surface, even though the amount of
solubility in pure water at the temperatures at the iron present may be very small.