Page 128 - Semiconductor For Micro- and Nanotechnology An Introduction For Engineers
P. 128
Free and Bound Electrons, Dimensionality Effects
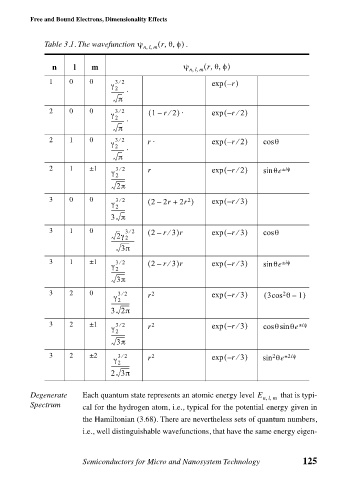
Table 3.1. The wavefunction ψ
,,
,,
,,
n l m nlm ( r θφ) ψ . nlm ( r θφ)
,,
1 0 0 32 r – ( )
⁄
γ 2 exp
---------- ⋅
π
⁄
⁄
⁄
2 0 0 32 ( 1 r 2) ⋅ – ( r 2)
γ 2 – exp
---------- ⋅
π
2 1 0 32 r ⋅ – ( r 2) θ
⁄
⁄
γ 2 exp cos
---------- ⋅
π
⁄
2 1 ±1 32 r exp – ( r 2) ± iφ
⁄
γ 2 sin θe
----------
2π
3 0 0 32 ( 2r ) exp – ( r 3)
⁄
⁄
2
–
γ 2 22r +
----------
3 π
3 1 0 32 ( 2 r 3)r exp – ( r 3⁄ ) cos θ
⁄
⁄
–
2γ 2
-----------------
3π
3 1 ±1 32 ( 2 r 3)r exp – ( r 3) ± iφ
⁄
⁄
⁄
–
γ 2 sin θe
----------
3π
3 2 0 32 2 exp – ( r 3) ( 3cos θ 1)
⁄
⁄
2
γ 2 r –
--------------
32π
3 2 ±1 32 2 – ( r 3) ± iφ
⁄
⁄
γ 2 r exp cos θsin θe
----------
3π
⁄
3 2 ±2 32 2 – ( r 3) 2 ± 2iφ
⁄
γ r exp sin θe
2
--------------
23π
Degenerate Each quantum state represents an atomic energy level E nlm that is typi-
,,
Spectrum cal for the hydrogen atom, i.e., typical for the potential energy given in
the Hamiltonian (3.68). There are nevertheless sets of quantum numbers,
i.e., well distinguishable wavefunctions, that have the same energy eigen-
Semiconductors for Micro and Nanosystem Technology 125