Page 235 - Shale Shakers Drilling Fluid Systems
P. 235
SOLIDS DEWATERINC 217
content. The only use for this water is in the Although more expensive, buffered phosphoric is
mud system. the most desirable because it is relatively safe.
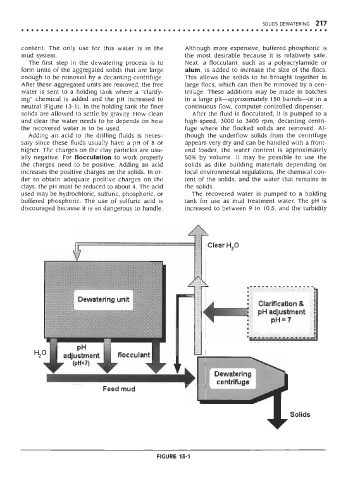
The first step in the dewatering process is to Next, a flocculant, such as a polyacrylamide or
form units of the aggregated solids that are large alum, is added to increase the size of the floes.
enough to be removed by a decanting centrifuge. This allows the solids to be brought together in
After these aggregated units are removed, the free large floes, which can then be removed by a cen-
water is sent to a holding tank where a "clarify- trifuge. These additions may be made in batches
ing" chemical is added and the pH increased to in a large pit—approximately 150 barrels—or in a
neutral (Figure 13-1). In the holding tank the finer continuous flow, computer-controlled dispenser.
solids are allowed to settle by gravity. How clean After the fluid is flocculated, it is pumped to a
and clear the water needs to be depends on how high-speed, 3000 to 3400 rpm, decanting centri-
the recovered water is to be used. fuge where the flocked solids are removed. Al-
Adding an acid to the drilling fluids is neces- though the underflow solids from the centrifuge
sary since these fluids usually have a pH of 8 or appears very dry and can be handled with a front-
higher. The charges on the clay particles are usu- end loader, the water content is approximately
ally negative. For flocculation to work properly 50% by volume. It may be possible to use the
the charges need to be positive. Adding an acid solids as dike building materials depending on
increases the positive charges on the solids. In or- local environmental regulations, the chemical con-
der to obtain adequate positive charges on the tent of the solids, and the water that remains in
clays, the pH must be reduced to about 4. The acid the solids.
used may be hydrochloric, sulfuric, phosphoric, or The recovered water is pumped to a holding
buffered phosphoric. The use of sulfuric acid is tank for use as mud treatment water. The pH is
discouraged because it is so dangerous to handle. increased to between 9 to 10.5, and the turbidity
FIGURE 13-1