Page 240 - Shale Shakers Drilling Fluid Systems
P. 240
222 SHALE SHAKERS AND DRILLING FLUID SYSTEMS
to approximately five times their original size (Fig-
ure 13-3). The particles are absorbing water that
migrates across the boundary and through the oil.
Once the small particles-in-water swell, almost
all will burst or explode simultaneously. This process
is known as microscopic eruption (Figure 13-4).
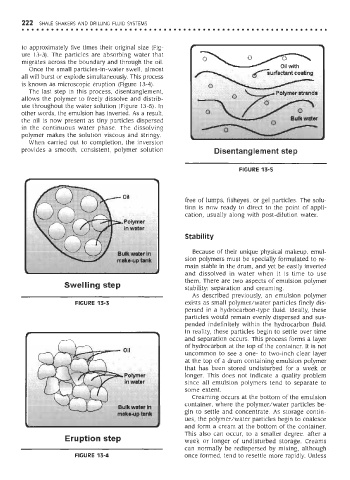
The last step in this process, disentanglement,
allows the polymer to freely dissolve and distrib-
ute throughout the water solution (Figure 13-5). In
other words, the emulsion has inverted. As a result,
the oil is now present as tiny particles dispersed
in the continuous water phase. The dissolving
polymer makes the solution viscous and stringy.
When carried out to completion, the inversion
provides a smooth, consistent, polymer solution
FIGURE 13-5
free of lumps, fisheyes, or gel particles. The solu-
tion is now ready to direct to the point of appli-
cation, usually along with post-dilution water.
Stability
Because of their unique physical makeup, emul-
sion polymers must be specially formulated to re-
main stable in the drum, and yet be easily inverted
and dissolved in water when it is time to use
them. There are two aspects of emulsion polymer
stability: separation and creaming.
As described previously, an emulsion polymer
FIGURE 13-3 exists as small polymer/water particles finely dis-
persed in a hydrocarbon-type fluid. Ideally, these
particles would remain evenly dispersed and sus-
pended indefinitely within the hydrocarbon fluid.
In reality, these particles begin to settle over time
and separation occurs. This process forms a layer
of hydrocarbon at the top of the container. It is not
uncommon to see a one- to two-inch clear layer
at the top of a drum containing emulsion polymer
that has been stored undisturbed for a week or
longer. This does not indicate a quality problem
since all emulsion polymers tend to separate to
some extent.
Creaming occurs at the bottom of the emulsion
container, where the polymer/water particles be-
gin to settle and concentrate. As storage contin-
ues, the polymer/water particles begin to coalesce
and form a cream at the bottom of the container.
This also can occur, to a smaller degree, after a
week or longer of undisturbed storage. Creams
can normally be redispersed by mixing, although
FIGURE 13-4 once formed, tend to resettle more rapidly. Unless