Page 239 - Shale Shakers Drilling Fluid Systems
P. 239
SOLIDS DEWATERINC 221
treatment polymers. These polymers are the surfactants help the polymer to "invert" into a con-
fastest growing physical form in the water treat- tinuous aqueous phase upon dilution of the neat
ment market. product. This makes emulsion polymers easy to
Years ago, when polyacrylamides were first de- dissolve and prepare for application. The surfactants
veloped, only dry polymer was available. Dry poly- must be balanced to provide this desired effect.
mers were difficult to handle due to dusting, as
well as slow and difficult to dissolve. Next, liquid
solution polymers were developed, which were Inversion
mainly dilutions of dry polymers. Liquid solution
polymers eliminated the dusting problem associated The term "inversion" refers to the change from
with dry polymers, however, because of their dilute a water-in-oil emulsion to an oil-in-water emul-
nature, they were extremely freight sensitive. sion. Inversion allows the coiled polymer chains
Emulsion polymers, developed only 12 to 14 years to uncoil and expand into the water phase. This
ago, were designed to provide the advantages of is accomplished by mixing neat emulsion polymer
both dry and liquid solution polymers, without any and water under prescribed mixing and dilution
of their associated problems. In this respect, emul- conditions. Optimum inversion of emulsion polymers
sion polymers can be viewed as the third genera- is the key to achieving maximum performance.
tion of water-soluble polymer technology. The complete inversion of an emulsion polymer
Because of their liquid form, emulsion polymers is actually a complex process on a microscopic
are free from dusting, easy to handle, store, dilute, scale, involving several sequential steps. In order
and feed. Like dry polymers, emulsion polymers of occurrence, these steps are:
are concentrated (normally 25% to 35% active)
and, therefore, are not freight sensitive and can 1. Dispersion
be shipped economically long distances from the 2. Swelling
manufacturing plant. 3. Eruption
Another important characteristic of emulsion 4. Disentanglement
polymers is that they can be manufactured to pos-
sess any ionic character: anionic, cationic, or non- When an emulsion polymer is added to water,
ionic. Also, practically any degree of ionic charge the first thing that occurs is dispersion of the
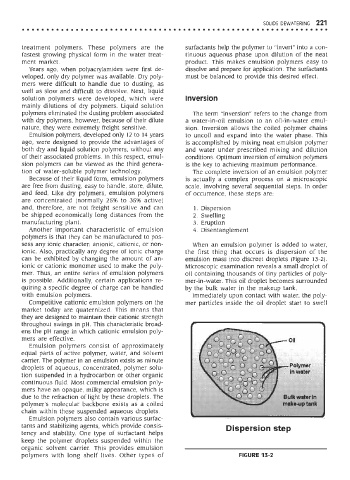
can be exhibited by changing the amount of an- emulsion mass into discreet droplets (Figure 13-2).
ionic or cationic monomer used to make the poly- Microscopic examination reveals a small droplet of
mer. Thus, an entire series of emulsion polymers oil containing thousands of tiny particles of poly-
is possible. Additionally, certain applications re- mer-in-water. This oil droplet becomes surrounded
quiring a specific degree of charge can be handled by the bulk water in the makeup tank.
with emulsion polymers. Immediately upon contact with water, the poly-
Competitive cationic emulsion polymers on the mer particles inside the oil droplet start to swell
market today are quaternized. This means that
they are designed to maintain their cationic strength
throughout swings in pH. This characteristic broad-
ens the pH range in which cationic emulsion poly-
mers are effective.
Emulsion polymers consist of approximately
equal parts of active polymer, water, and solvent
carrier. The polymer in an emulsion exists as minute
droplets of aqueous, concentrated, polymer solu-
tion suspended in a hydrocarbon or other organic
continuous fluid. Most commercial emulsion poly-
mers have an opaque, milky appearance, which is
due to the refraction of light by these droplets. The
polymer's molecular backbone exists as a coiled
chain within these suspended aqueous droplets.
Emulsion polymers also contain various surfac-
tants and stabilizing agents, which provide consis-
tency and stability. One type of surfactant helps
keep the polymer droplets suspended within the
organic solvent carrier. This provides emulsion
polymers with long shelf lives. Other types of FIGURE 13-2