Page 315 - Software and Systems Requirements Engineering in Practice
P. 315
A
n
r
d
s
i
y
a
l
a
1
r
t
e
a
z
H
1
:
o
d
M
t
n
g
i
e
l
a
n
d
a
s
r
e
h
T
h
C C h a p t e r 1 1 : H a z a r d A n a l y s i s a n d T h r e a t M o d e l i n g 277 277
p
a
• Hazard analysis Identification of a substance, activity, or
condition as potentially posing a risk to human health or
safety.
• Risk assessment The process of identifying hazards and
quantifying or qualifying the degree of risk they pose for
exposed individuals, populations, or resources (severity) and
the likelihood that the hazard will occur (probability of
occurrence). The term also refers to a document containing
the explanation of how the assessment process is applied to
individual activities or conditions.
• Safety-critical system A system that has been designated
by a regulatory body as needing a hazard analysis before
being put into operation.
• Severity The actual categorization of severity is usually
domain specific. For example, the categorizations for the
Food and Drug Administration (FDA) and the Federal Transit
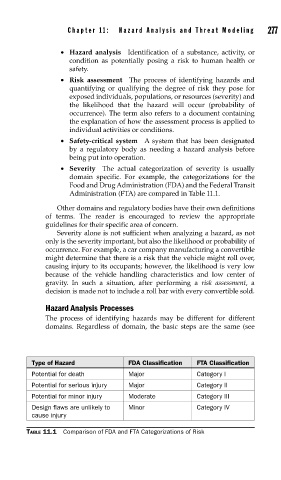
Administration (FTA) are compared in Table 11.1.
Other domains and regulatory bodies have their own definitions
of terms. The reader is encouraged to review the appropriate
guidelines for their specific area of concern.
Severity alone is not sufficient when analyzing a hazard, as not
only is the severity important, but also the likelihood or probability of
occurrence. For example, a car company manufacturing a convertible
might determine that there is a risk that the vehicle might roll over,
causing injury to its occupants; however, the likelihood is very low
because of the vehicle handling characteristics and low center of
gravity. In such a situation, after performing a risk assessment, a
decision is made not to include a roll bar with every convertible sold.
Hazard Analysis Processes
The process of identifying hazards may be different for different
domains. Regardless of domain, the basic steps are the same (see
Type of Hazard FDA Classification FTA Classification
Potential for death Major Category I
Potential for serious injury Major Category II
Potential for minor injury Moderate Category III
Design flaws are unlikely to Minor Category IV
cause injury
TABLE 11.1 Comparison of FDA and FTA Categorizations of Risk