Page 150 - Soil Degradation, Conservation and Remediation
P. 150
5.3 Acidification 139
Effective Neutralizing Value (ENV)
The ENV is the fraction of the liming material’s CCE that will react with soil acidity
in the first year of application. The ENV is calculated by multiplying the CCE with
the fineness of the material. For example, a liming material with CCE of 90 % and
a fineness of 0.86 has an ENV of 90 × 0.86 = 77.4. The neutralizing value of a liming
material is its capacity to neutralize acidity. The higher the NV, the more pure is the
product. Pure calcium carbonate (pure limestone) is taken as the standard with an
NV of 100. The neutralizing value of commercial limestone is usually between 96
and 98. Other liming materials are more reactive than limestone and therefore have
higher neutralizing values, for example, hydrated lime and burnt lime.
5.3.3.3 Mechanism of Lime Action
Lime dissolves slowly in soil solution to release Ca and bicarbonate ions:
CaCO + HO CO = Ca 2+ + 2 HCO −
+
3 2 2 3
2+
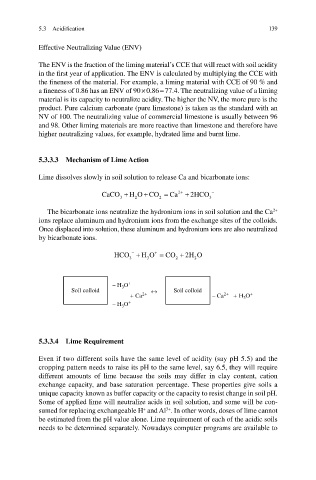
The bicarbonate ions neutralize the hydronium ions in soil solution and the Ca
ions replace aluminum and hydronium ions from the exchange sites of the colloids.
Once displaced into solution, these aluminum and hydronium ions are also neutralized
by bicarbonate ions.
+
HCO + H O = CO + 2 H O
−
3 3 2 2
− H O +
3
Soil colloid ↔ Soil colloid
+ Ca 2+ − Ca 2+ + H O +
3
− H O +
3
5.3.3.4 Lime Requirement
Even if two different soils have the same level of acidity (say pH 5.5) and the
cropping pattern needs to raise its pH to the same level, say 6.5, they will require
different amounts of lime because the soils may differ in clay content, cation
exchange capacity, and base saturation percentage. These properties give soils a
unique capacity known as buffer capacity or the capacity to resist change in soil pH.
Some of applied lime will neutralize acids in soil solution, and some will be con-
3+
sumed for replacing exchangeable H and Al . In other words, doses of lime cannot
+
be estimated from the pH value alone. Lime requirement of each of the acidic soils
needs to be determined separately. Nowadays computer programs are available to