Page 191 - Soil Degradation, Conservation and Remediation
P. 191
180 6 Soil Pollution
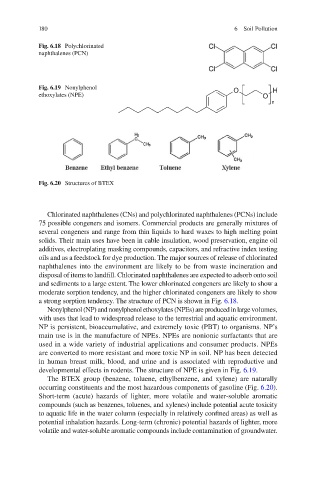
Fig. 6.18 Polychlorinated
naphthalenes (PCN)
Fig. 6.19 Nonylphenol
ethoxylates (NPE)
Fig. 6.20 Structures of BTEX
Chlorinated naphthalenes (CNs) and polychlorinated naphthalenes (PCNs) include
75 possible congeners and isomers. Commercial products are generally mixtures of
several congeners and range from thin liquids to hard waxes to high melting point
solids. Their main uses have been in cable insulation, wood preservation, engine oil
additives, electroplating masking compounds, capacitors, and refractive index testing
oils and as a feedstock for dye production. The major sources of release of chlorinated
naphthalenes into the environment are likely to be from waste incineration and
disposal of items to landfill. Chlorinated naphthalenes are expected to adsorb onto soil
and sediments to a large extent. The lower chlorinated congeners are likely to show a
moderate sorption tendency, and the higher chlorinated congeners are likely to show
a strong sorption tendency. The structure of PCN is shown in Fig. 6.18 .
Nonylphenol (NP) and nonylphenol ethoxylates (NPEs) are produced in large volumes,
with uses that lead to widespread release to the terrestrial and aquatic environment.
NP is persistent, bioaccumulative, and extremely toxic (PBT) to organisms. NP’s
main use is in the manufacture of NPEs. NPEs are nonionic surfactants that are
used in a wide variety of industrial applications and consumer products. NPEs
are converted to more resistant and more toxic NP in soil. NP has been detected
in human breast milk, blood, and urine and is associated with reproductive and
developmental effects in rodents. The structure of NPE is given in Fig. 6.19 .
The BTEX group (benzene, toluene, ethylbenzene, and xylene) are naturally
occurring constituents and the most hazardous components of gasoline (Fig. 6.20 ).
Short-term (acute) hazards of lighter, more volatile and water-soluble aromatic
compounds (such as benzenes, toluenes, and xylenes) include potential acute toxicity
to aquatic life in the water column (especially in relatively confined areas) as well as
potential inhalation hazards. Long-term (chronic) potential hazards of lighter, more
volatile and water-soluble aromatic compounds include contamination of groundwater.