Page 121 - Soil and water contamination, 2nd edition
P. 121
108 Soil and Water Contamination
100
6642 6642 6642
80
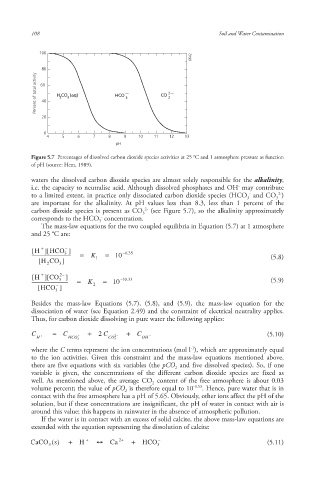
Percent of total activity 60 H CO (aq) HCO — 3 CO 2—
3
3
2
40
20
0
4 5 6 7 8 9 10 11 12 13
pH
Figure 5.7 Percentages of dissolved carbon dioxide species activities at 25 °C and 1 atmosphere pressure as function
of pH (source: Hem, 1989).
waters the dissolved carbon dioxide species are almost solely responsible for the alkalinity ,
-
i.e. the capacity to neutralise acid . Although dissolved phosphates and OH may contribute
-
2-
to a limited extent, in practice only dissociated carbon dioxide species (HCO and CO )
3 3
are important for the alkalinity. At pH values less than 8.3, less than 1 percent of the
2-
carbon dioxide species is present as CO (see Figure 5.7), so the alkalinity approximately
3
-
corresponds to the HCO concentration.
3
The mass-law equations for the two coupled equilibria in Equation (5.7) at 1 atmosphere
and 25 °C are:
[H ][HCO 3 ] . 6 35
K 1 10 (5.8)
[H 2 CO 3 ]
[H ][CO 2 ]
3 K 10 10 . 33 (5.9)
[HCO ] 2
3
Besides the mass-law Equations (5.7), (5.8), and (5.9), the mass-law equation for the
dissociation of water (see Equation 2.49) and the constraint of electrical neutrality applies.
Thus, for carbon dioxide dissolving in pure water the following applies:
C C 2 C C (5.10)
H HCO 3 CO 3 2 OH
-1
where the C terms represent the ion concentrations (mol l ), which are approximately equal
to the ion activities. Given this constraint and the mass-law equations mentioned above,
there are five equations with six variables (the pCO and five dissolved species). So, if one
2
variable is given, the concentrations of the different carbon dioxide species are fixed as
well. As mentioned above, the average CO content of the free atmosphere is about 0.03
2
volume percent; the value of pCO is therefore equal to 10 -3.53 . Hence, pure water that is in
2
contact with the free atmosphere has a pH of 5.65. Obviously, other ions affect the pH of the
solution, but if these concentrations are insignificant, the pH of water in contact with air is
around this value; this happens in rainwater in the absence of atmospheric pollution.
If the water is in contact with an excess of solid calcite , the above mass-law equations are
extended with the equation representing the dissolution of calcite:
CaCO (s ) + H + Ca 2 + + HCO (5.11)
3 3
10/1/2013 6:44:27 PM
Soil and Water.indd 120 10/1/2013 6:44:27 PM
Soil and Water.indd 120