Page 117 - Soil and water contamination, 2nd edition
P. 117
104 Soil and Water Contamination
Aluminium occurs in substantial amounts in many silicate, igneous, and sedimentary
rock minerals, such as feldspars , amphiboles, micas , and clay minerals (see Section 4.2.3). On
weathering of these minerals, Al is usually retained in newly formed solid clay minerals (see
also Figure 4.2), some of which may be considerably enriched in Al. Gibbsite (Al(OH) ) is
3
a common mineral in acidic podsolic soils in temperate and subarctic regions and in highly
weathered soils in tropical regions. Gibbsite is also the major constituent of bauxite, the ore
from which aluminium is produced.
The solubility of Al is strongly controlled by the solution pH . This pH control on the
different forms of Al species can be elucidated by the following equilibrium equation:
[Al( H 2 O) 6 ] + 3 [Al( H 2 O () 5 OH ] ) + 2 + H +
+
[Al( H O () OH) ] + 2 H +
2 4 2
0 + (5.5)
[Al( H 2 O () 3 OH) 3 ] + 3 H
[Al( H O () OH) ] + 4 H +
2 2 4
[Al( H 2 O)( OH) 3 ] 2 + 5 H +
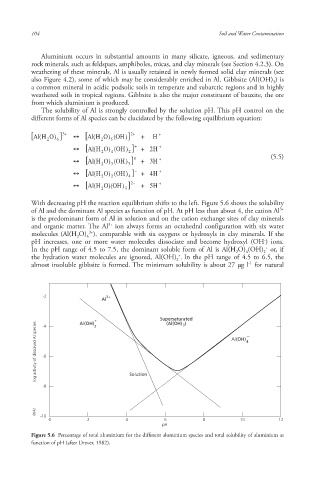
With decreasing pH the reaction equilibrium shifts to the left. Figure 5.6 shows the solubility
3+
of Al and the dominant Al species as function of pH. At pH less than about 4, the cation Al
is the predominant form of Al in solution and on the cation exchange sites of clay minerals
3+
and organic matter . The Al ion always forms an octahedral configuration with six water
3+
molecules (Al(H O) ), comparable with six oxygens or hydroxyls in clay minerals. If the
2 6
-
pH increases, one or more water molecules dissociate and become hydroxyl (OH ) ions.
+
In the pH range of 4.5 to 7.5, the dominant soluble form of Al is Al(H O) (OH) or, if
2
2
4
+
the hydration water molecules are ignored, Al(OH) . In the pH range of 4.5 to 6.5, the
2
-1
almost insoluble gibbsite is formed. The minimum solubility is about 27 μg l for natural
-2 3+
Al
Supersaturated
+ 2 (Al(OH) )
log activity of dissolved Al species -6 Al(OH) —
Al(OH)
3
-4
4
-8 Solution
6642 6642 6642 -10
0 2 4 6 8 10 12
pH
Figure 5.6 Percentage of total aluminium for the different aluminium species and total solubility of aluminium as
function of pH (after Drever, 1982).
10/1/2013 6:44:26 PM
Soil and Water.indd 116 10/1/2013 6:44:26 PM
Soil and Water.indd 116