Page 115 - Soil and water contamination, 2nd edition
P. 115
102 Soil and Water Contamination
1.40
6642
Water oxidised
1.20
1.00
0.80
0.60 t MnO 2
2+
Mn
0.40
t MnOOH
0.20
Eh (Volt)
0.00 Mn O
3 4
3
-0.20
MnCOH
-0.40 55 mg/l Mn(OH) 2 -
5.5 mg/l Mn(OH) 3
0.55 mg/l
55 μg/l MnS
-0.60
5.5 μg/l
Dissolved Manganese
5.5 μg/l
-0.80
55 μg/l
Water reduced
-1.00
0 2 4 6 8 10 12 14
pH
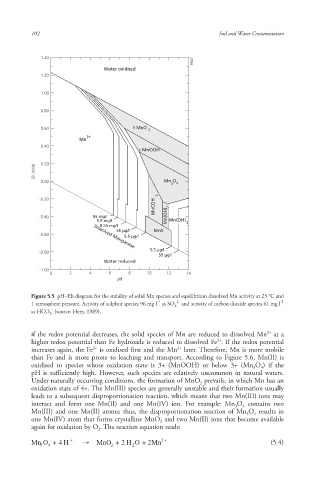
Figure 5.5 pH–Eh diagram for the stability of solid Mn species and equilibrium dissolved Mn activity at 25 °C and
-1 2- -1
1 atmosphere pressure. Activity of sulphur species 96 mg l as SO and activity of carbon dioxide species 61 mg l
4
-
as HCO (source: Hem, 1989).
3
2+
if the redox potential decreases, the solid species of Mn are reduced to dissolved Mn at a
2+
higher redox potential than Fe hydroxide is reduced to dissolved Fe . If the redox potential
2+
2+
increases again, the Fe is oxidised first and the Mn later. Therefore, Mn is more mobile
than Fe and is more prone to leaching and transport. According to Figure 5.6, Mn(II) is
oxidised to species whose oxidation state is 3+ (MnOOH) or below 3+ (Mn O ) if the
3 4
pH is sufficiently high. However, such species are relatively uncommon in natural waters.
Under naturally occurring conditions, the formation of MnO prevails, in which Mn has an
2
oxidation state of 4+. The Mn(III) species are generally unstable and their formation usually
leads to a subsequent disproportionation reaction, which means that two Mn(III) ions may
interact and form one Mn(II) and one Mn(IV) ion. For example: Mn O contains two
3 4
Mn(III) and one Mn(II) atoms; thus, the disproportionation reaction of Mn O results in
3 4
one Mn(IV) atom that forms crystalline MnO and two Mn(II) ions that become available
2
again for oxidation by O . The reaction equation reads:
2
Mn 3 O 4 + 4 H + MnO 2 + 2 H 2 O + 2Mn 2 + (5.4)
10/1/2013 6:44:25 PM
Soil and Water.indd 114 10/1/2013 6:44:25 PM
Soil and Water.indd 114