Page 132 - Soil and water contamination, 2nd edition
P. 132
Nutrients 119
2 NO + 10 e + 12 H +
3
2 NO + 6 e + 8 H + + H O
2 2
NO2 + 4 e + 6 H + + 4 H O (6.3)
2
N O + 2 e + 2 H + + 5 H O
2 2
N + 6 H O
2 2
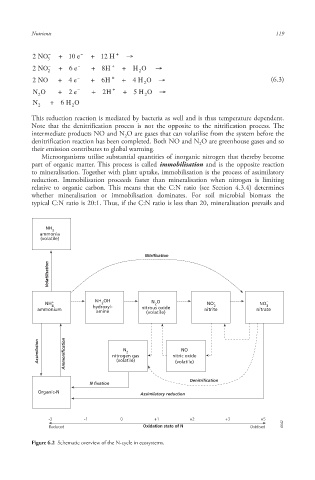
This reduction reaction is mediated by bacteria as well and is thus temperature dependent.
Note that the denitrification process is not the opposite to the nitrification process. The
intermediate products NO and N O are gases that can volatilise from the system before the
2
denitrification reaction has been completed. Both NO and N O are greenhouse gases and so
2
their emission contributes to global warming.
Microorganisms utilise substantial quantities of inorganic nitrogen that thereby become
part of organic matter . This process is called immobilisation and is the opposite reaction
to mineralisation . Together with plant uptake , immobilisation is the process of assimilatory
reduction. Immobilisation proceeds faster than mineralisation when nitrogen is limiting
relative to organic carbon . This means that the C:N ratio (see Section 4.3.4) determines
whether mineralisation or immobilisation dominates. For soil microbial biomass the
typical C:N ratio is 20:1. Thus, if the C:N ratio is less than 20, mineralisation prevails and
NH
3
ammonia
(volatile)
Nitrification
Volatilisation
2
NH + NH OH N O NO - NO -
2
4
2
3
ammonium hydroxyl- nitrous oxide nitrite nitrate
amine (volatile)
Assimilation Ammonification nitrogen gas nitric oxide
N
NO
2
(volatile)
(volatile)
Denitrification
N fixation
Organic-N Assimilatory reduction
-3 -1 0 +1 +2 +3 +5
Reduced Oxidation state of N Oxidised 6642 6642 6642
Figure 6.2 Schematic overview of the N-cycle in ecosystems.
10/1/2013 6:44:31 PM
Soil and Water.indd 131 10/1/2013 6:44:31 PM
Soil and Water.indd 131