Page 135 - Soil and water contamination, 2nd edition
P. 135
122 Soil and Water Contamination
100
6642 6642 6642
80
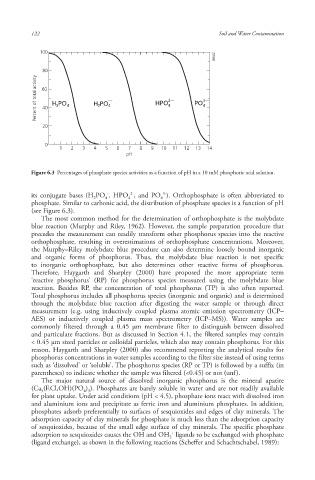
Percent of total activity 60 H PO 43 H PO 42 — HPO 2— PO 3—
4
4
40
20
0
1 2 3 4 5 6 7 8 9 10 11 12 13 14
pH
Figure 6.3 Percentages of phosphate species activities as a function of pH in a 10 mM phosphoric acid solution.
3-
-
2-
its conjugate bases (H PO , HPO , and PO ). Orthophosphate is often abbreviated to
2 4 4 4
phosphate. Similar to carbonic acid , the distribution of phosphate species is a function of pH
(see Figure 6.3).
The most common method for the determination of orthophosphate is the molybdate
blue reaction (Murphy and Riley, 1962). However, the sample preparation procedure that
precedes the measurement can readily transform other phosphorus species into the reactive
orthophosphate, resulting in overestimations of orthophosphate concentrations. Moreover,
the Murphy–Riley molybdate blue procedure can also determine loosely bound inorganic
and organic forms of phosphorus. Thus, the molybdate blue reaction is not specific
to inorganic orthophosphate, but also determines other reactive forms of phosphorus.
Therefore, Haygarth and Sharpley (2000) have proposed the more appropriate term
‘reactive phosphorus ’ (RP) for phosphorus species measured using the molybdate blue
reaction. Besides RP, the concentration of total phosphorus (TP) is also often reported.
Total phosphorus includes all phosphorus species (inorganic and organic) and is determined
through the molybdate blue reaction after digesting the water sample or through direct
measurement (e.g. using inductively coupled plasma atomic emission spectrometry (ICP–
AES) or inductively coupled plasma mass spectrometry (ICP–MS)). Water samples are
commonly filtered through a 0.45 μm membrane filter to distinguish between dissolved
and particulate fractions. But as discussed in Section 4.1, the filtered samples may contain
< 0.45 μm sized particles or colloidal particles, which also may contain phosphorus. For this
reason, Haygarth and Sharpley (2000) also recommend reporting the analytical results for
phosphorus concentrations in water samples according to the filter size instead of using terms
such as ‘dissolved’ or ‘soluble’. The phosphorus species (RP or TP) is followed by a suffix (in
parentheses) to indicate whether the sample was filtered (<0.45) or not (unf).
The major natural source of dissolved inorganic phosphorus is the mineral apatite
(Ca (F,Cl,OH)(PO ) ). Phosphates are barely soluble in water and are not readily available
5 4 3
for plant uptake . Under acid conditions (pH < 4.5), phosphate ions react with dissolved iron
and aluminium ions and precipitate as ferric iron and aluminium phosphates. In addition,
phosphates adsorb preferentially to surfaces of sesquioxides and edges of clay minerals . The
adsorption capacity of clay minerals for phosphate is much less than the adsorption capacity
of sesquioxides, because of the small edge surface of clay minerals. The specific phosphate
+
adsorption to sesquioxides causes the OH and OH ligands to be exchanged with phosphate
2
(ligand exchange), as shown in the following reactions (Scheffer and Schachtschabel, 1989):
10/1/2013 6:44:31 PM
Soil and Water.indd 134 10/1/2013 6:44:31 PM
Soil and Water.indd 134