Page 136 - Soil and water contamination, 2nd edition
P. 136
Nutrients 123
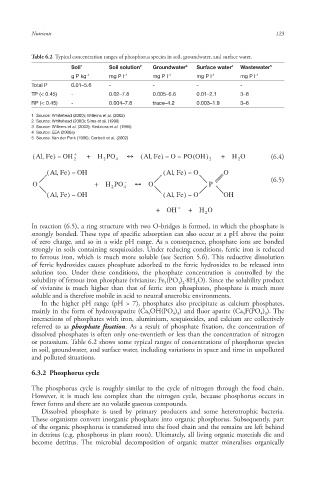
Table 6.2 Typical concentration ranges of phosphorus species in soil, groundwater, and surface water.
Soil 1 Soil solution 2 Groundwater 3 Surface water 4 Wastewater 5
g P kg -1 mg P l -1 mg P l -1 mg P l -1 mg P l -1
Total P 0.01–5.6 - - - -
TP (< 0.45) - 0.02–7.8 0.005–6.6 0.01–2.1 3–8
RP (< 0.45) - 0.004–7.8 trace–4.2 0.003–1.9 3–6
1 Source: Whitehead (2000); Willems et al. (2002)
2 Source: Whitehead (2000); Sims et al. (1998)
3 Source: Willems et al. (2002); Kedziora et al. (1995)
4 Source: EEA (2006a)
5 Source: Van der Perk (1996); Corbett et al. (2002)
( Al, Fe) OH + + H PO ( Al, Fe) O PO( OH) + H O (6.4)
2 2 4 2 2
( Al, Fe) OH ( Al, Fe) O O
(6.5)
O + H 2 PO 4 O P
( Al, Fe) OH ( Al, Fe) O OH
+ OH + H O
2
In reaction (6.5), a ring structure with two O-bridges is formed, in which the phosphate is
strongly bonded. These type of specific adsorption can also occur at a pH above the point
of zero charge , and so in a wide pH range. As a consequence, phosphate ions are bonded
strongly in soils containing sesquioxides . Under reducing conditions, ferric iron is reduced
to ferrous iron , which is much more soluble (see Section 5.6). This reductive dissolution
of ferric hydroxides causes phosphate adsorbed to the ferric hydroxides to be released into
solution too. Under these conditions, the phosphate concentration is controlled by the
solubility of ferrous iron phosphate (vivianite ; Fe (PO ) ⋅8H O). Since the solubility product
3 4 2 2
of vivianite is much higher than that of ferric iron phosphates, phosphate is much more
soluble and is therefore mobile in acid to neutral anaerobic environments.
In the higher pH range (pH > 7), phosphates also precipitate as calcium phosphates,
mainly in the form of hydroxyapatite (Ca OH(PO ) ) and fluor apatite (Ca F(PO ) ). The
5 4 3 5 4 3
interactions of phosphates with iron , aluminium , sesquioxides , and calcium are collectively
referred to as phosphate fixation . As a result of phosphate fixation, the concentration of
dissolved phosphates is often only one-twentieth or less than the concentration of nitrogen
or potassium . Table 6.2 shows some typical ranges of concentrations of phosphorus species
in soil, groundwater, and surface water, including variations in space and time in unpolluted
and polluted situations.
6.3.2 Phosphorus cycle
The phosphorus cycle is roughly similar to the cycle of nitrogen through the food chain.
However, it is much less complex than the nitrogen cycle, because phosphorus occurs in
fewer forms and there are no volatile gaseous compounds.
Dissolved phosphate is used by primary producers and some heterotrophic bacteria.
These organisms convert inorganic phosphate into organic phosphorus . Subsequently, part
of the organic phosphorus is transferred into the food chain and the remains are left behind
in detritus (e.g. phosphorus in plant roots). Ultimately, all living organic materials die and
become detritus. The microbial decomposition of organic matter mineralises organically
10/1/2013 6:44:32 PM
Soil and Water.indd 135 10/1/2013 6:44:32 PM
Soil and Water.indd 135