Page 142 - Soil and water contamination, 2nd edition
P. 142
Heavy metals 129
from building materials (roofs, gutters, pipes, lead slabs), deposition of contaminated
river sediments, and direct domestic or industrial discharges and disposals. Computers,
televisions, and other electronic equipment contain an array of trace materials, including
lead, mercury , cadmium , and arsenic . In the past twenty years, the releases of heavy metals
to the environment has been considerably reduced as a result of improved waste air and
water purification techniques, waste recycling, and the implementation of more stringent
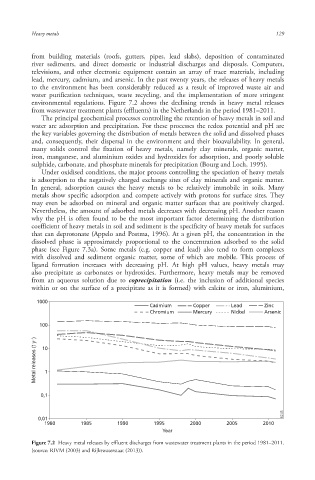
environmental regulations. Figure 7.2 shows the declining trends in heavy metal releases
from wastewater treatment plants (effluents) in the Netherlands in the period 1981–2011.
The principal geochemical processes controlling the retention of heavy metals in soil and
water are adsorption and precipitation. For these processes the redox potential and pH are
the key variables governing the distribution of metals between the solid and dissolved phases
and, consequently, their dispersal in the environment and their bioavailability. In general,
many solids control the fixation of heavy metals, namely clay minerals, organic matter,
iron, manganese, and aluminium oxides and hydroxides for adsorption, and poorly soluble
sulphide , carbonate , and phosphate minerals for precipitation (Bourg and Loch, 1995).
Under oxidised conditions, the major process controlling the speciation of heavy metals
is adsorption to the negatively charged exchange sites of clay minerals and organic matter .
In general, adsorption causes the heavy metals to be relatively immobile in soils. Many
metals show specific adsorption and compete actively with protons for surface sites. They
may even be adsorbed on mineral and organic matter surfaces that are positively charged.
Nevertheless, the amount of adsorbed metals decreases with decreasing pH . Another reason
why the pH is often found to be the most important factor determining the distribution
coefficient of heavy metals in soil and sediment is the specificity of heavy metals for surfaces
that can deprotonate (Appelo and Postma, 1996). At a given pH, the concentration in the
dissolved phase is approximately proportional to the concentration adsorbed to the solid
phase (see Figure 7.3a). Some metals (e.g. copper and lead ) also tend to form complexes
with dissolved and sediment organic matter, some of which are mobile. This process of
ligand formation increases with decreasing pH. At high pH values, heavy metals may
also precipitate as carbonates or hydroxides. Furthermore, heavy metals may be removed
from an aqueous solution due to coprecipitation (i.e. the inclusion of additional species
within or on the surface of a precipitate as it is formed) with calcite or iron , aluminium ,
Figure 7.2 Heavy metal releases by effluent discharges from wastewater treatment plants in the period 1981–2011.
(source: RIVM (2003) and Rijkswaterstaat (2013)).
10/1/2013 6:44:33 PM
Soil and Water.indd 141
Soil and Water.indd 141 10/1/2013 6:44:33 PM