Page 143 - Soil and water contamination, 2nd edition
P. 143
130 Soil and Water Contamination
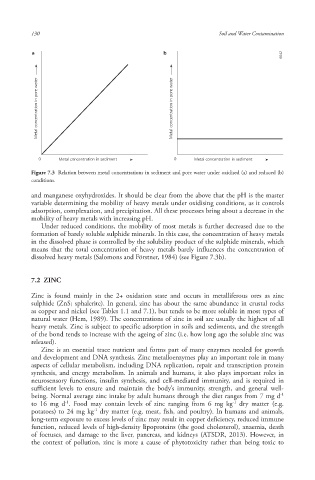
a b
6642 6642 6642
Metal concentration in pore water Metal concentration in pore water
0 Metal concentration in sediment 0 Metal concentration in sediment
Figure 7.3 Relation between metal concentrations in sediment and pore water under oxidised (a) and reduced (b)
conditions.
and manganese oxyhydroxides . It should be clear from the above that the pH is the master
variable determining the mobility of heavy metals under oxidising conditions, as it controls
adsorption, complexation , and precipitation. All these processes bring about a decrease in the
mobility of heavy metals with increasing pH.
Under reduced conditions, the mobility of most metals is further decreased due to the
formation of barely soluble sulphide minerals. In this case, the concentration of heavy metals
in the dissolved phase is controlled by the solubility product of the sulphide minerals, which
means that the total concentration of heavy metals barely influences the concentration of
dissolved heavy metals (Salomons and Förstner, 1984) (see Figure 7.3b).
7.2 ZINC
Zinc is found mainly in the 2+ oxidation state and occurs in metalliferous ores as zinc
sulphide (ZnS; sphalerite). In general, zinc has about the same abundance in crustal rocks
as copper and nickel (see Tables 1.1 and 7.1), but tends to be more soluble in most types of
natural water (Hem, 1989). The concentrations of zinc in soil are usually the highest of all
heavy metals . Zinc is subject to specific adsorption in soils and sediments, and the strength
of the bond tends to increase with the ageing of zinc (i.e. how long ago the soluble zinc was
released).
Zinc is an essential trace nutrient and forms part of many enzymes needed for growth
and development and DNA synthesis. Zinc metalloenzymes play an important role in many
aspects of cellular metabolism, including DNA replication, repair and transcription protein
synthesis, and energy metabolism. In animals and humans, it also plays important roles in
neurosensory functions, insulin synthesis, and cell-mediated immunity, and is required in
sufficient levels to ensure and maintain the body’s immunity, strength, and general well-
-1
being. Normal average zinc intake by adult humans through the diet ranges from 7 mg d
-1
-1
to 16 mg d . Food may contain levels of zinc ranging from 6 mg kg dry matter (e.g.
-1
potatoes) to 24 mg kg dry matter (e.g. meat, fish, and poultry). In humans and animals,
long-term exposure to excess levels of zinc may result in copper deficiency, reduced immune
function, reduced levels of high-density lipoproteins (the good cholesterol), anaemia, death
of foetuses, and damage to the liver, pancreas, and kidneys (ATSDR, 2013). However, in
the context of pollution, zinc is more a cause of phytotoxicity rather than being toxic to
10/1/2013 6:44:33 PM
Soil and Water.indd 142 10/1/2013 6:44:33 PM
Soil and Water.indd 142