Page 31 - Soil and water contamination, 2nd edition
P. 31
18 Soil and Water Contamination
[ x m (2.2)
]
i i i
-1
where [x ] = the activity of an ion i (mol l ), γ = the activity coefficient [-], and m is the
i i i
-1
molar concentration of the ion i (mol l ). The activity coefficient γ depends on the ionic
i
strength , which is a measure of the total concentration of dissolved ions:
2
0
I 5 m z (2.3)
.
i i
where I = the ionic strength and z = the charge of the ion i. The ionic strength of fresh water
i
is usually less than 0.02 and that of ocean water is about 0.7. The activity coefficient depends
not only on the ionic strength but also on the specific charge of the ion, and on temperature
and pressure. Activity coefficients are tabulated in chemical textbooks as function of ionic
strength and specific charge, or can be estimated from several activity models, such as the
extended Debye–Hückel equation:
2
A z I (2.4)
log i i
1 Ba I
i
where A and B are temperature -dependent constants and a = the radius of the hydrated ion
i
[L]. The values for A, B, and a are listed in Table 2.3. The extended Debye–Hückel equation
i
can be used to estimate values of the activity coefficient to a maximum ionic strength of
-1
about 0.1 or a total dissolved solids concentration of approximately 5000 mg l (Domineco
and Schwarz, 1997). However, it is worth noting that in natural fresh waters the activity
coefficient is usually close to one and the effect of ionic strength may be neglected for
approximate calculations. In saline waters, such as ocean water, it is usually necessary to use
activity coefficients in equilibrium calculations. Studies of more saline waters, such as brines,
require other more sophisticated activity models, for example the Pitzer model (Pitzer, 1981).
For further descriptions of chemical equilibrium modelling, see standard chemical textbooks,
such as Stumm and Morgan (1996), Drever (2000), or Faure (1998).
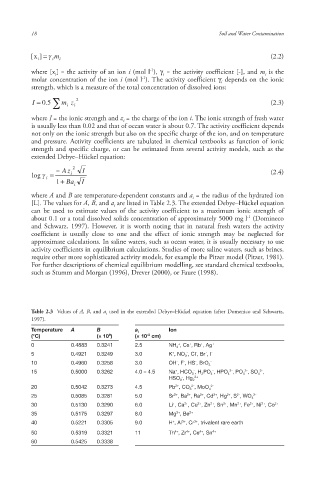
Table 2.3 Values of A, B, and a i used in the extended Debye–Hückel equation (after Domenico and Schwartz,
1997).
Temperature A B a i Ion
-8
8
(°C) (× 10 ) (× 10 cm)
+
+
+
0 0.4883 0.3241 2.5 NH 4 , Cs , Rb , Ag +
-
+
-
-
5 0.4921 0.3249 3.0 K , NO 3 , Cl , Br , I -
-
-
10 0.4960 0.3258 3.0 OH , F , HS , BrO 3 -
-
2-
-
2-
+
3-
-
15 0.5000 0.3262 4.0 – 4.5 Na , HCO 3 , H 2 PO 4 , HPO 4 , PO 4 , SO 4 ,
- 2+
HSO 3 , Hg 2
2-
2+
20 0.5042 0.3273 4.5 Pb , CO 3 , MoO 4 2-
2+
25 0.5085 0.3281 5.0 Sr , Ba , Ra , Cd , Hg , S , WO 4 2-
2+
2+
2+
2+
2-
2+
2+
2+
2+
+
2+
2+
2+
30 0.5130 0.3290 6.0 Li , Ca , Cu , Zn , Sn , Mn , Fe , Ni , Co 2+
2+
35 0.5175 0.3297 8.0 Mg , Be 2+
+
40 0.5221 0.3305 9.0 H , Al , Cr , trivalent rare earth
3+
3+
4+
4+
4+
50 0.5319 0.3321 11 Th , Zr , Ce , Sn 4+
60 0.5425 0.3338
10/1/2013 6:44:06 PM
Soil and Water.indd 30
Soil and Water.indd 30 10/1/2013 6:44:06 PM