Page 35 - Soil and water contamination, 2nd edition
P. 35
22 Soil and Water Contamination
energy (i.e. a change in enthalpy) to convert a solid into a liquid and, subsequently, into a gas
with no temperature change. Conversely, during phase transitions towards a phase with less
entropy, this energy is released again. This energy is called latent heat; it becomes manifest
as an increase in temperature when the entropy decreases, for instance during condensation.
The specific latent heat of fusion of a substance is the amount of heat required to convert a
unit mass of a solid into a liquid without a change in temperature; the specific latent heat of
vaporisation is the amount of heat required to convert a unit mass of a liquid into a vapour
without a change in temperature. The latent heats expressed per mole of a substance are
called enthalpies of fusion and vaporisation, respectively. Specific latent heats or enthalpies
of fusion and vaporisation are tabulated in many physical and chemical textbooks. Phase
transitions from or to the aqueous phase are commonly considered as chemical reactions.
The thermodynamics of these transitions will be dealt with in Section 2.6.
2.5.3 Partition coefficient
It has often been observed experimentally that at small concentrations the ratio of
concentrations in two phases in equilibrium is constant. This constant is also called the
partition coefficient :
C phase1
K (2.9)
C
phase2
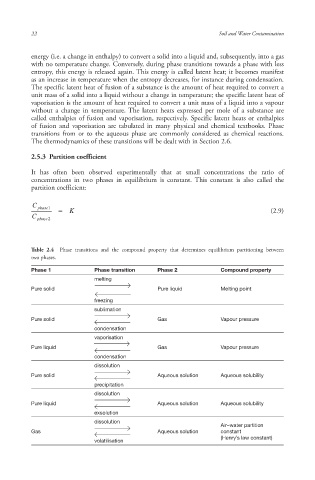
Table 2.4 Phase transitions and the compound property that determines equilibrium partitioning between
two phases.
Phase 1 Phase transition Phase 2 Compound property
melting
Pure solid Pure liquid Melting point
freezing
sublimation
Pure solid Gas Vapour pressure
condensation
vaporisation
Pure liquid Gas Vapour pressure
condensation
dissolution
Pure solid Aqueous solution Aqueous solubility
precipitation
dissolution
Pure liquid Aqueous solution Aqueous solubility
exsolution
dissolution
Air–water partition
Gas Aqueous solution constant
(Henry’s law constant )
volatilisation
10/1/2013 6:44:10 PM
Soil and Water.indd 34 10/1/2013 6:44:10 PM
Soil and Water.indd 34