Page 32 - Soil and water contamination, 2nd edition
P. 32
Basic environmental chemistry 19
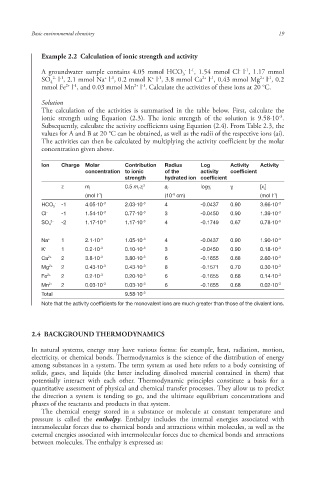
Example 2.2 Calculation of ionic strength and activity
-
-1
-
-1
A groundwater sample contains 4.05 mmol HCO l , 1.54 mmol Cl l , 1.17 mmol
3
+ -1
+ -1
2+ -1
2+ -1
2- -1
SO l , 2.1 mmol Na l , 0.2 mmol K l , 3.8 mmol Ca l , 0.43 mmol Mg l , 0.2
4
2+ -1
2+ -1
mmol Fe l , and 0.03 mmol Mn l . Calculate the activities of these ions at 20 °C.
Solution
The calculation of the activities is summarised in the table below. First, calculate the
-3
ionic strength using Equation (2.3). The ionic strength of the solution is 9.58·10 .
Subsequently, calculate the activity coefficient s using Equation (2.4). From Table 2.3, the
values for A and B at 20 °C can be obtained, as well as the radii of the respective ions (ai).
The activities can then be calculated by multiplying the activity coefficient by the molar
concentration given above.
Ion Charge Molar Contribution Radius Log Activity Activity
concentration to ionic of the activity coefficient
strength hydrated ion coefficient
z m i 0.5 m i ·z i 2 a i logγ i γ i [x i ]
-8
-1
-1
(mol l ) (10 cm) (mol l )
- -1 4.05·10 -3 2.03·10 -3 4 -0.0437 0.90 3.66·10 -3
HCO 3
Cl - -1 1.54·10 -3 0.77·10 -3 3 -0.0450 0.90 1.39·10 -3
2- -2 1.17·10 -3 1.17·10 -3 4 -0.1749 0.67 0.78·10 -3
SO 4
Na + 1 2.1·10 -3 1.05·10 -3 4 -0.0437 0.90 1.90·10 -3
K + 1 0.2·10 -3 0.10·10 -3 3 -0.0450 0.90 0.18·10 -3
Ca 2+ 2 3.8·10 -3 3.80·10 -3 6 -0.1655 0.68 2.60·10 -3
Mg 2+ 2 0.43·10 -3 0.43·10 -3 8 -0.1571 0.70 0.30·10 -3
Fe 2+ 2 0.2·10 -3 0.20·10 -3 6 -0.1655 0.68 0.14·10 -3
Mn 2+ 2 0.03·10 -3 0.03·10 -3 6 -0.1655 0.68 0.02·10 -3
Total 9.58·10 -3
Note that the activity coefficient s for the monovalent ions are much greater than those of the divalent ions.
2.4 BACKGROUND THERMODYNAMICS
In natural systems, energy may have various forms: for example, heat, radiation , motion,
electricity, or chemical bonds. Thermodynamics is the science of the distribution of energy
among substances in a system. The term system as used here refers to a body consisting of
solids, gases, and liquids (the latter including dissolved material contained in them) that
potentially interact with each other. Thermodynamic principles constitute a basis for a
quantitative assessment of physical and chemical transfer processes. They allow us to predict
the direction a system is tending to go, and the ultimate equilibrium concentrations and
phases of the reactants and products in that system.
The chemical energy stored in a substance or molecule at constant temperature and
pressure is called the enthalpy . Enthalpy includes the internal energies associated with
intramolecular forces due to chemical bonds and attractions within molecules, as well as the
external energies associated with intermolecular forces due to chemical bonds and attractions
between molecules. The enthalpy is expressed as:
10/1/2013 6:44:09 PM
Soil and Water.indd 31 10/1/2013 6:44:09 PM
Soil and Water.indd 31