Page 381 - Soil and water contamination, 2nd edition
P. 381
368 Soil and Water Contamination
mg P l -1
0 2 4 6
0 September
November
March
5 May
August
Depth (cm) 10
15
6642 6642 6642
20
-1
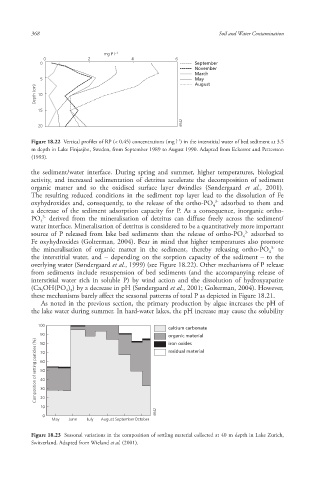
Figure 18.22 Vertical profiles of RP (< 0.45) concentrations (mg l ) in the interstitial water of bed sediment at 3.5
m depth in Lake Finjasjön, Sweden, from September 1989 to August 1990. Adapted from Eckerrot and Pettersson
(1993).
the sediment/water interface. During spring and summer, higher temperatures, biological
activity, and increased sedimentation of detritus accelerate the decomposition of sediment
organic matter and so the oxidised surface layer dwindles (Søndergaard et al., 2001).
The resulting reduced conditions in the sediment top layer lead to the dissolution of Fe
3-
oxyhydroxides and, consequently, to the release of the ortho-PO adsorbed to them and
4
a decrease of the sediment adsorption capacity for P. As a consequence, inorganic ortho-
3-
PO derived from the mineralisation of detritus can diffuse freely across the sediment/
4
water interface. Mineralisation of detritus is considered to be a quantitatively more important
3-
source of P released from lake bed sediments than the release of ortho-PO adsorbed to
4
Fe oxyhydroxides (Golterman, 2004). Bear in mind that higher temperatures also promote
3-
the mineralisation of organic matter in the sediment, thereby releasing ortho-PO to
4
the interstitial water, and – depending on the sorption capacity of the sediment – to the
overlying water (Søndergaard et al., 1999) (see Figure 18.22). Other mechanisms of P release
from sediments include resuspension of bed sediments (and the accompanying release of
interstitial water rich in soluble P) by wind action and the dissolution of hydroxyapatite
(Ca OH(PO ) ) by a decrease in pH (Søndergaard et al., 2001; Golterman, 2004). However,
5 4 3
these mechanisms barely affect the seasonal patterns of total P as depicted in Figure 18.21.
As noted in the previous section, the primary production by algae increases the pH of
the lake water during summer. In hard-water lakes , the pH increase may cause the solubility
100
calcium carbonate
90 organic material
Composition of setting particles (%) 70
iron oxides
80
residual material
60
50
40
30
20
10
6642 6642 6642
0
May June July August SeptemberOctober
Figure 18.23 Seasonal variations in the composition of settling material collected at 40 m depth in Lake Zurich,
Switzerland. Adapted from Wieland et al. (2001).
10/1/2013 6:47:50 PM
Soil and Water.indd 380
Soil and Water.indd 380 10/1/2013 6:47:50 PM