Page 62 - Soil and water contamination, 2nd edition
P. 62
Basic environmental chemistry 49
12. A buffered solution is prepared by mixing 500 ml of 0.5 M acetic acid (CH COOH) and
3
500 ml of 0.5 M sodium acetate (NaCH COO).
3
a. Calculate the pH of the buffered solution given the pK of acetic acid = 4.75.
a
b. Calculate the pH after 0.05 mol of HCl has been added to the buffered solution.
2+
13. The presence of dissolved iron (Fe ) is an appropriate indicator of the redox conditions
in groundwater.
a. Give the half reaction for the reduction of iron hydroxide (Fe(OH) ) to dissolved
3
2+
Fe .
b. What is the effect of lowering the pH on the solubility of Fe(OH) ?
3
2+
c. Give the relationship of Eh versus dissolved Fe concentration and pH given the log
K for the above half reaction = 16.43 (Hint: calculate the relationship between pe and
2+
Fe and pH first).
2+
2+
Draw the line between Fe(OH) and Fe in a pH–Eh diagram at a fixed dissolved Fe
3
-1
concentration of 10 -6.00 mol l .
2+
d. In which natural environments is iron hydroxide reduced to dissolved Fe (compare
Figure 2.3)?
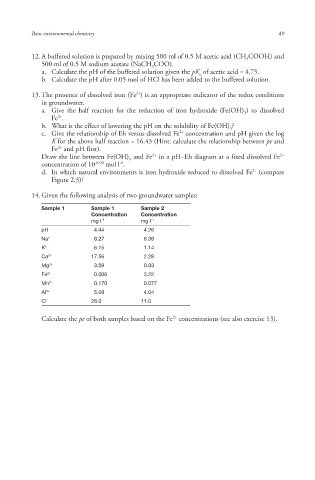
14. Given the following analysis of two groundwater samples:
Sample 1 Sample 1 Sample 2
Concentration Concentration
mg l -1 mg l -1
pH 4.44 4.26
Na + 8.27 6.38
K + 6.15 1.14
Ca 2+ 17.56 2.28
Mg 2+ 3.09 0.93
Fe 2+ 0.006 3.22
Mn 2+ 0.170 0.077
Al 3+ 5.08 4.04
Cl - 28.0 11.0
2+
Calculate the pe of both samples based on the Fe concentrations (see also exercise 13).
10/1/2013 6:44:21 PM
Soil and Water.indd 61 10/1/2013 6:44:21 PM
Soil and Water.indd 61