Page 86 - Soil and water contamination, 2nd edition
P. 86
Solid phase constituents 73
Secondary minerals have been formed by the precipitation or recrystallisation of elements
that have been released by the chemical weathering of primary minerals and normally have
particle sizes smaller than 2 μm (also referred to as the clay fraction, see also Figure 4.1).
The inorganic components may also be classified according to their solubility . Very soluble
minerals, such as nitrates, halides, and some sulphates, are usually only present as secondary
precipitates in soils under arid conditions (i.e. low soil moisture contents). Carbonates and
gypsum are intermediate soluble minerals and are found in soil as primary minerals in parent
sedimentary bedrock material or as secondary precipitates . Under natural conditions, gypsum
only precipitates out under the influence of evaporation . The dissolution and precipitation
of carbonates depend on the pH and CO pressure in the dissolved phase (see Section 3.6).
2
Sulphides (e.g. pyrite ) are secondary minerals, which are formed under reducing conditions
when sulphate is reduced to sulphide (see Section 5.7). Sulphides are practically insoluble,
but under oxic conditions sulphide may oxidise to sulphate and dissolve. Many silicates , such
as quartz (note that quartz is also a silicate!), tectosilicates, nesosilicates, inosilicates, micas ,
and serpentine, are primary minerals and can be considered as the remains of igneous and
metamorphic bedrock material. They have a very low reactivity , since they combine very low
solubility with a small specific surface area. The remainder of the minerals listed in Table
4.1 are iron (Fe) and aluminium (Al) oxides/hydroxides , and clay minerals . These secondary
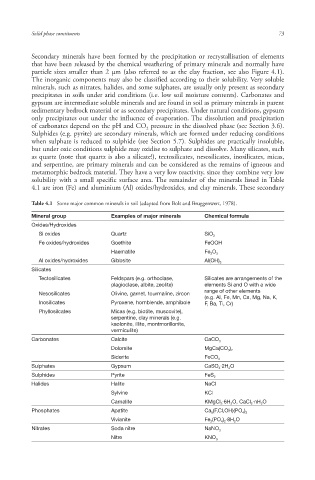
Table 4.1 Some major common minerals in soil (adapted from Bolt and Bruggenwert, 1978).
Mineral group Examples of major minerals Chemical formula
Oxides/Hydroxides
Si oxides Quartz SiO 2
Fe oxides/hydroxides Goethite FeOOH
Haematite Fe 2 O 3
Al oxides/hydroxides Gibbsite Al(OH) 3
Silicates
Tectosilicates Feldspars (e.g. orthoclase, Silicates are arrangements of the
plagioclase, albite, zeolite) elements Si and O with a wide
range of other elements
Nesosilicates Olivine, garnet, tourmaline, zircon
(e.g. Al, Fe, Mn, Ca, Mg, Na, K,
Inosilicates Pyroxene , hornblende, amphibole F, Ba, Ti, Cr)
Phyllosilcates Micas (e.g. biotite, muscovite),
serpentine, clay minerals (e.g.
kaolonite, illite, montmorillonite,
vermiculite )
Carbonates Calcite CaCO 3
Dolomite MgCa(CO 3 ) 2
Siderite FeCO 3
.
Sulphates Gypsum CaSO 4 2H 2 O
Sulphides Pyrite FeS 2
Halides Halite NaCl
Sylvine KCl
.
.
Carnalite KMgCl 3 6H 2 O, CaCl 2 nH 2 O
Phosphates Apatite Ca 5 (F,Cl,OH)(PO 4 ) 3
.
Vivianite Fe 3 (PO 4 ) 2 8H 2 O
Nitrates Soda nitre NaNO 3
Nitre KNO 3
10/1/2013 6:44:22 PM
Soil and Water.indd 85
Soil and Water.indd 85 10/1/2013 6:44:22 PM