Page 90 - Soil and water contamination, 2nd edition
P. 90
Solid phase constituents 77
6642 6642 6642
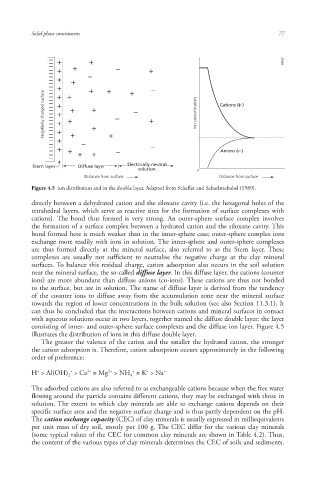
Negatively charged surface Ion concentration Cations ( )
Anions ( )
Stern layer Diffuse layer Electrically neutral
solution 0
Distance from surface Distance from surface
Figure 4.5 Ion distribution and in the double layer. Adapted from Scheffer and Schachtschabel (1989).
directly between a dehydrated cation and the siloxane cavity (i.e. the hexagonal holes of the
tetrahedral layers, which serve as reactive sites for the formation of surface complexes with
cations). The bond thus formed is very strong. An outer-sphere surface complex involves
the formation of a surface complex between a hydrated cation and the siloxane cavity. This
bond formed here is much weaker than in the inner-sphere case; outer-sphere complex ions
exchange more readily with ions in solution . The inner-sphere and outer-sphere complexes
are thus formed directly at the mineral surface, also referred to as the Stern layer. These
complexes are usually not sufficient to neutralise the negative charge at the clay mineral
surfaces. To balance this residual charge, cation adsorption also occurs in the soil solution
near the mineral surface, the so-called diffuse laye r. In this diffuse layer, the cations (counter
ions) are more abundant than diffuse anions (co-ions). These cations are thus not bonded
to the surface, but are in solution. The name of diffuse layer is derived from the tendency
of the counter ions to diffuse away from the accumulation zone near the mineral surface
towards the region of lower concentrations in the bulk solution (see also Section 11.3.1). It
can thus be concluded that the interactions between cations and mineral surfaces in contact
with aqueous solutions occur in two layers, together named the diffuse double layer: the layer
consisting of inner- and outer-sphere surface complexes and the diffuse ion layer. Figure 4.5
illustrates the distribution of ions in this diffuse double layer.
The greater the valence of the cation and the smaller the hydrated cation, the stronger
the cation adsorption is. Therefore, cation adsorption occurs approximately in the following
order of preference:
+
+
+
+
2+
2+
H > Al(OH) > Ca ≈ Mg > NH ≈ K > Na +
2 4
The adsorbed cations are also referred to as exchangeable cations because when the free water
flowing around the particle contains different cations, they may be exchanged with those in
solution. The extent to which clay minerals are able to exchange cations depends on their
specific surface area and the negative surface charge and is thus partly dependent on the pH.
The cation exchange capacity (CEC ) of clay minerals is usually expressed in milliequivalents
per unit mass of dry soil, mostly per 100 g. The CEC differ for the various clay minerals
(some typical values of the CEC for common clay minerals are shown in Table 4.2). Thus,
the content of the various types of clay minerals determines the CEC of soils and sediments.
10/1/2013 6:44:22 PM
Soil and Water.indd 89 10/1/2013 6:44:22 PM
Soil and Water.indd 89